Introduction
Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) are the most common types of autoimmune thyroid disease (AITD) in children and adolescents.
1) Both disorders are characterized by the presence of pathognomonic autoantibodies to thyroid antigens. Most patients with GD have anti-thyrotropin receptor (TSHR) antibodies, and most patients with HT have anti-thyroid peroxidase (TPO) antibodies and anti-thyroglobulin (Tg) antibodies.
2)
Traditionally, GD and HT are considered to be two distinct AITD with different pathophysiologies. However, recent studies hypothesize that they constitute one continuous disease with opposite manifestations.
3) From this point of view, both HT and GD are multifaceted; they can represent both hypo- and hyperthyroidism, with possible transformation from GD to HT or vice versa.
4,5) Herein, we report two pediatric cases with a conversion from HT to GD. We review the mechanism of this rare phenomenon with regards to the concept of a continuum involving autoimmune antibodies to the thyroid gland.
Go to :

Case Reports
Case 1
A 10-year-old girl had hypothyroidism for 3 years due to HT and had been taking levothyroxine (0.05 mg/day). Over the past month, she developed new-onset symptoms associated with hyperthyroidism, such as fatigue, goiter, and unintentional weight loss (approximately 3-4 kg/month).
She visited another clinic, where her laboratory results revealed free T4 levels of 3.3 ng/dL (0.8-1.9), thyroid-stimulating hormone (TSH) levels of 0.005 μIU/mL (0.4-4.2), TSHR-binding inhibitory immuno-globulin (TBII, assessed by competitive binding assay) levels of 3.07 IU/L (positive ≥1.75), anti-Tg antibody of 509.41 U/mL (negative <4.11), and anti-TPO antibody of 122.61 U/mL (negative <5.61) (
Table 1). She had a right-mid thyroid mass 22.1×14.4 mm in size, with heterogenous and poorly defined findings on ultrasonography (
Fig. 1). Fine-needle aspiration cytology revealed nodular goiter (adenomatous hyperplasia) with Hurthle cell change. She was diagnosed with GD, and treatment with propranolol (10 mg/day) and methimazole (MM) (10 mg, b.i.d.) was initiated.
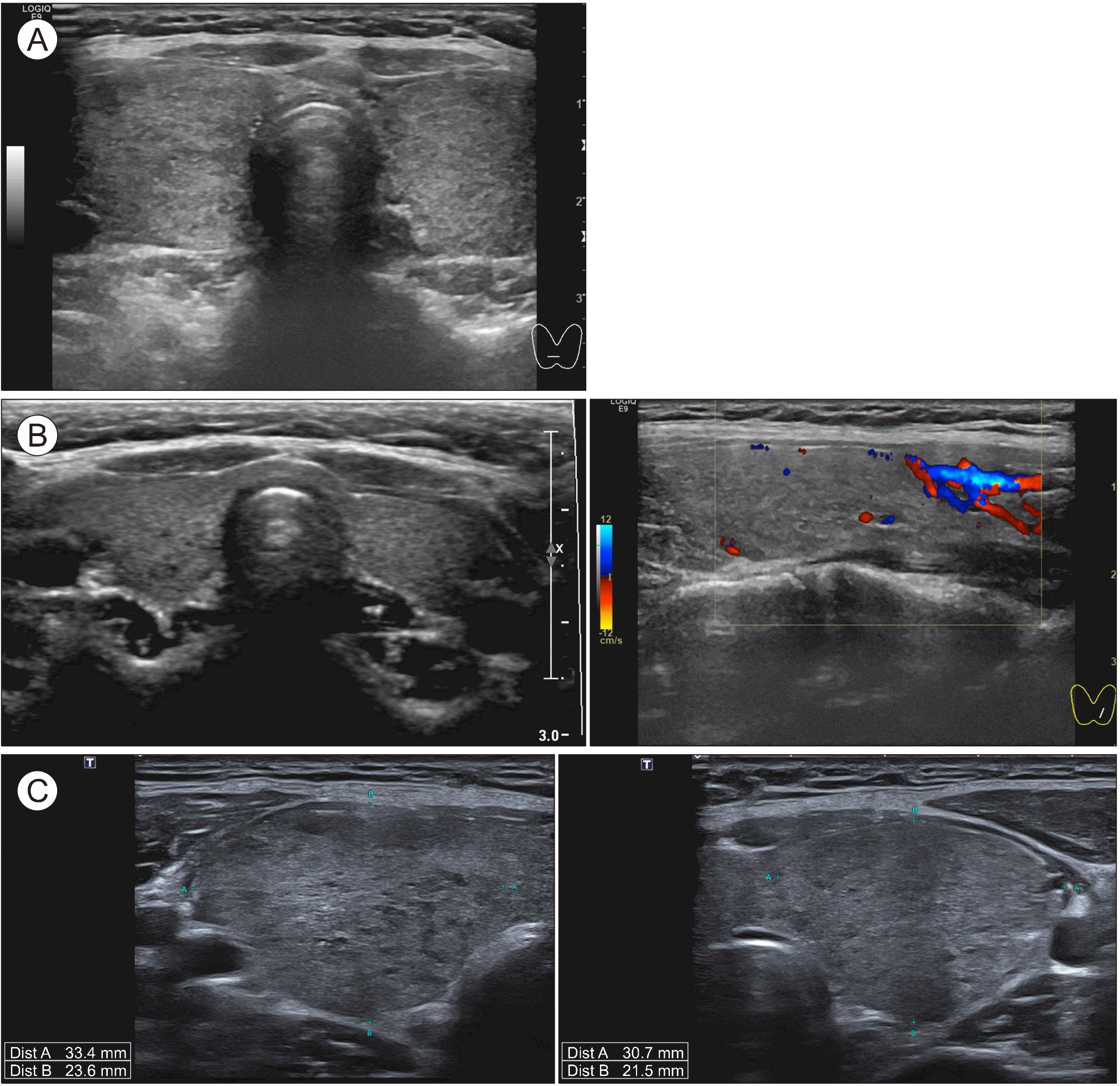 | Fig. 1Thyroid ultrasound. Patient 1: (A) At 7 years and 6 months of age, Hashimoto’s disease was diagnosed. Sonography findings include decreased echogenicity in both thyroid lobes. Multiple benign-cysts and -nodules are detected. (B) At age 10 years and 9 months, during the Graves’ disease period, sonography showed a diffuse goiter and increased vascularity. (C) At age 12 years and 2 months, sonography shows goiter and heterogenous echotexture consistent with Graves’ disease. At this stage, thyroid function was not under control, despite medication with methimazole. 
|
Table 1
Progression from Hashimoto’s thyroiditis to Graves’ disease: Case 1
|
Age |
TSH μU/mL
(0.3-4) |
Free T4 ng/dL
(0.77-1.94) |
T3 ng/mL
(0.75-1.9) |
TBII IU/L
(0-1.5) |
Anti-TPO antibody U/mL
(0-60) |
Anti-Tg antibody U/mL
(0-60) |
LT dose (mg) |
MM dose (mg) |
|
7 y, 6 m |
5.07 |
1.33 |
1.09 |
Nd*
|
1.15 |
167.44 |
0.05†
|
- |
|
10 y, 5 m‡
|
0.005 |
3.3 |
Nd |
3.07 |
122.61 |
509.41 |
D/C§
|
10 b.i.d.∥
|
|
11 y, 2 m |
6.30 |
1.19 |
1.25 |
1.40 |
168.82 |
Nd |
D/C |
2.5 q.d.¶
|
|
11 y, 10 m |
0.02 |
3.55 |
2.72 |
79.34 |
1626.8 |
Nd |
D/C |
10 b.i.d.**
|
|
12 y, 10 m |
0.01 |
1.75 |
1.87 |
Nd |
Nd |
Nd |
D/C |
15 b.i.d. |
|
12 y, 11 m |
0.01 |
1.83 |
2.23 |
Nd |
Nd |
Nd |
D/C |
15 b.i.d. |

Since hypothyroidism onset, she continued to be sero-positive for anti-TPO and anti-Tg antibodies. We did not test for TBII when HT was diagnosed; however, after diagnosis with GD, the serology test showed TBII level of 3.07 IU/L (positive ≥1.75). After 7 months of treatment with MM, test results were transiently sero-negative for TBII (1.40 IU/L [0-1.5]). Apart from this, she continued to be sero-positive for TBII. Hyperthyroidism was not adequately controlled for 17 months, and her MM dosage was increased to 15 mg b.i.d.. The patient continues to be treated with MM for GD for 28 months.
Case 2
An 11-year-old girl had hypothyroidism for 2 months due to HT, for which she underwent levothyroxine replacement therapy (0.05 mg/day).
At HT diagnosis, she had no symptoms other than goiter (
Fig. 2). After 2 months of follow-up, her thyroid hormone levels increased; the free T4 levels were 2.35 ng/dL (0.77-1.94), T3 levels were 2.40 ng/mL (0.75-1.9), and TSH levels were 0.07 μIU/mL (0.3-4) (
Table 2). Subsequently, the levothyroxine dose was reduced to half for 1 month and later stopped due to a constant rise in T3 (3.75 ng/mL) and free T4 (3.49 ng/mL) and decreased TSH levels (0.001 μIU/mL). Despite the dose adjustment, her laboratory test results persistently showed elevated T3 levels of 3.25 ng/mL (0.75-1.9) and free T4 levels of 3.40 ng/mL (0.77-1.94) and decreased TSH levels of 0.001 μIU/mL (0.3-4), as well as positive TBII at 34.37 IU/L (0-1.5) and positive anti-TPO antibody 286.01 U/mL (0-60). There were no symptoms corresponding to hyper-thyroidism. She was diagnosed with GD 2 months after the detection of elevated thyroid hormone levels. She required thyrostatic treatment (MM 7.5 g b.i.d.). She continued to take MM for approximately 20 months, after which it was stopped due to an elevated TSH levels to 65.97 μIU/mL. At that time, her anti-TPO antibody level was 3334.7 U/mL, anti-Tg antibody was 114.11 U/mL, and TBII level was 3.09 IU/L. Since discontinuation of treatment with MM, she has been in a euthyroid state for 1 year.
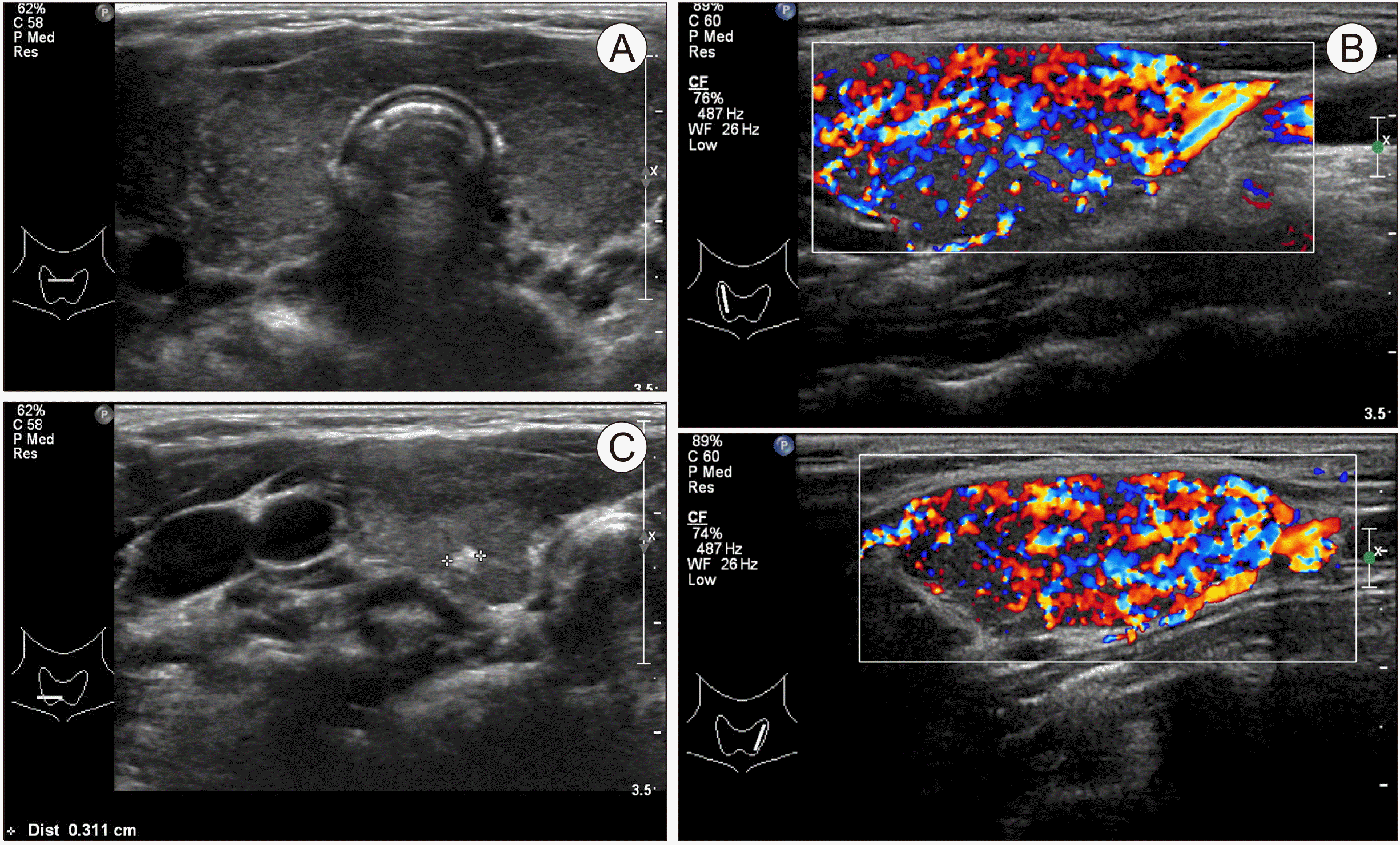 | Fig. 2Thyroid ultrasound. Patient 2: at age 11 years and 6 months, diagnosed with Hashimoto’s disease was diagnosed. Sonography shows (A) a diffuse goiter with heterogenous echotexture and (B) increased vascularity. (C) At the lower pole, a 0.3 cm calcified nodule can be seen. 
|
Table 2
Progression from Hashimoto’s thyroiditis to Graves’ disease: Case 2
|
Age |
TSH μU/mL
(0.3-4) |
Free T4 ng/dL
(0.77-1.94) |
T3 ng/mL
(0.75-1.9) |
TBII IU/L
(0-1.5) |
Anti-TPO antibody U/mL
(0-60) |
Anti-Tg antibody U/mL
(0-60) |
LT dose (mg) |
MM dose (mg) |
|
11 y, 6 m |
26.72 |
0.75 |
1.36 |
Nd*
|
286.01 |
168.73 |
0.05 q.d.†
|
- |
|
11 y, 9 m |
0.07 |
2.35 |
2.40 |
Nd |
Nd |
Nd |
0.025 q.d. |
- |
|
11 y, 10 m |
0.001 |
3.49 |
3.75 |
Nd |
Nd |
Nd |
D/C‡
|
- |
|
11 y, 11 m |
0.001 |
3.40 |
3.25 |
34.37 |
281.83 |
Nd |
D/C |
7.5 b.i.d. |
|
13 y, 8 m |
65.97 |
0.81 |
1.48 |
3.09 |
Nd |
Nd |
D/C |
D/C |
|
13 y, 9 m |
3.45 |
1.22 |
1.71 |
Nd |
3334.7 |
114.11 |
D/C |
D/C |

Go to :

Discussion
Cases of HT following GD are rare in the pediatric population; however, the conversion from HT to GD is an even rarer phenomenon.
6) Both disorders are characterized by the presence of pathognomonic autoantibodies to thyroid antigens.
1) Increased levels of specific anti-thyroid autoantibodies support AITD diagnosis.
2) Anti-TSHR antibodies (TRAbs) are detected in approximately 90% of patients with GD and up to 20% of those with HT.
2) TRAbs function to stimulate, block, or induce apoptosis.
2) Balance between these subtypes of TRAbs is a key factor in the manifestation of GD.
2) Anti-TPO and anti-Tg antibodies are detected with a prevalence of >80% in HT and a prevalence of >50% in GD cases. These antibodies are considered to causes HT,
2) leading to chronic thyroid lymphocytic infiltration and thyroid cell damage.
1)
Most populations with GD have anti-TPO and anti-Tg antibodies as well as TRAbs. However, majority of people with HT have anti-TPO and anti-Tg antibodies only, without TRAbs. This may reduce the chances of conversion of HT to GD.
3) Another opinion is that very little thyroid tissue remains the target of TRAbs in HT.
7)
The mechanism of this transition is not well defined,
8) however, according to a recent literature review, the relationship between HT and GD is considered a spectrum of AITD.
3) One explanation is the possibility of intermolecular autoimmune response expansion resulting in responses to the TSH subunit A autoantigen of TSHR in patients with HT and a response to TPO and Tg in patients with GD.
3)
Several researchers have studied the relationship between levothyroxine dose in patients with HT and blood levels of anti-TPO antibodies and TBII and found strong correlations between them.
6,9) Upon treatment with levothyroxine, an increase in regulatory T (Treg) cells was observed, which allows for the normal immune function of Treg cells.
10) Autoantibody levels in the thyroid gland may be altered by specific factors, and hypothyroidism or hyperthyroidism manifestations are determined by the balance among these auto-antibodies.
11) Increased TRAb concentration and activities may be involved in the transformation from HT to GD.
12) According to the seven cases of transition from GD to HD reported by Takasu et al.
7) and the two cases reported herein, patients diagnosed with GD exhibited significant changes in serum autoantibody level. After transformation into GD, increased blood level of TRAb was noted.
In patients with HT, hashitoxicosis or levothyroxine overdose should be suspected if thyrotoxicosis occurs during levothyroxine treatment. Hashitoxicosis is defined by hyperthyroidism during the course of HT because of abrupt destruction of thyrocytes. In the hashitoxicosis phase, patients may develop goiter and hyperthyroidism but are negative for GD-specific TRAbs and positive for anti-TPO or anti-Tg anti-bodies.
12,13)
Although rare, there is a possibility of transition to GD; thus, it is better to test TRAb titer for accurate diagnosis and timely treatment.
Adult cases of transition from HT to GD have been reported sporadically. In 1990, Takasu et al.
7) introduced seven patients who transitioned from HT hypothyroidism to GD hyperthyroidism (five patients had transient GD hyperthyroidism, two patients had persistent GD hyperthyroidism) and one patient who showed persistent hypothyroidism despite positive thyroid stimulation antibodies (TSAbs). Among them, one patient with transient hyperthyroidism was initially TBII-positive and thyroid blocking antibody (TBAb)-positive. Takasu and Matsushita
9) postulated that TSAbs and TBAbs activities and the responsiveness of the thyroid to TSAbs are key factors influencing those changes.
Another 24 adult cases were reported in 2018 by Gonzalez-Aguilera et al.
11) who hypothesized that HT to GD conversion depends on two TSH receptor-binding antibodies (stimulating or blocking) acting on the thyroid. The result of stimulating or blocking either one depends on the affinity of the anti-TSH autoantibodies. It manifests as hypothyroidism or hyperthyroidism.
11)
In 2014, the first pediatric case was reported. Aversa et al.
14) presented a comparison of 35 patients diagnosed with GD with Down’s or Turner’s syndrome (age range 5.5-29.5) and 109 age-matched patients without Down’s or Turner’s syndrome (age range 9.2-16.8). Nine out of 35 (25.7%) and 4 out of 109 (3.7%) patients experienced a conversion from HT to GD in the former and latter groups, respectively. The authors concluded that the changes did not relate with thyroid function or LT treatment and considered that the mechanism of biological change comprises a change in the TRAbs activity from TBAbs in the HT phase to TSAbs when GD appears.
14)
These two cases extend the rare reports of GD following HT to include the pediatric age group.
This study had limitations. We did not test TRAbs of patients when they were diagnosed with HT. Moreover, TRAb levels were measured by competitive binding assay (TBII), not by function (stimulation, blocking, or induction of apoptosis).
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download