CASE
A 20-year-old female presented with intermittent epigastric pain for 8 months with no history of nausea, vomiting, altered bowel habits, fever, jaundice, anorexia, weight loss, or a history of similar pain in the past. General physical examination was normal. Abdominal examination revealed a small vague non-tender lump in the epigastric region.
The patient was evaluated for the symptoms 6 months before at another center. All the routine hematological investigations were normal, but serum amylase (422 U/L, normal range 28–100 U/L) and serum lipase (1,048 U/L, normal range < 67 U/L) levels were raised. Ultrasonography showed a well-defined cystic lesion of 7.4 cm × 7.3 cm in the pancreatic head suggesting a pancreatic pseudocyst. The rest of the pancreas and gallbladder were normal. Contrast-enhanced computed tomography (CECT) (
Fig. 1A,
1B) and magnetic resonance imaging (MRI), including magnetic resonance cholangiopancreatography (MRCP), (
Fig. 1C,
1D) revealed a well-defined cystic lesion (8 cm × 8 cm, wall thickness 3 mm) in relation to the pancreatic head, bulky pancreatic body and tail, and prominent main pancreatic duct. No evidence of any septa or solid component was noted within the cyst. Pancreatic pseudocyst was suggested with a differential of choledochal cyst and cystic neoplasm. The patient was managed conservatively as a case of pancreatic pseudocyst with partial symptomatic relief.
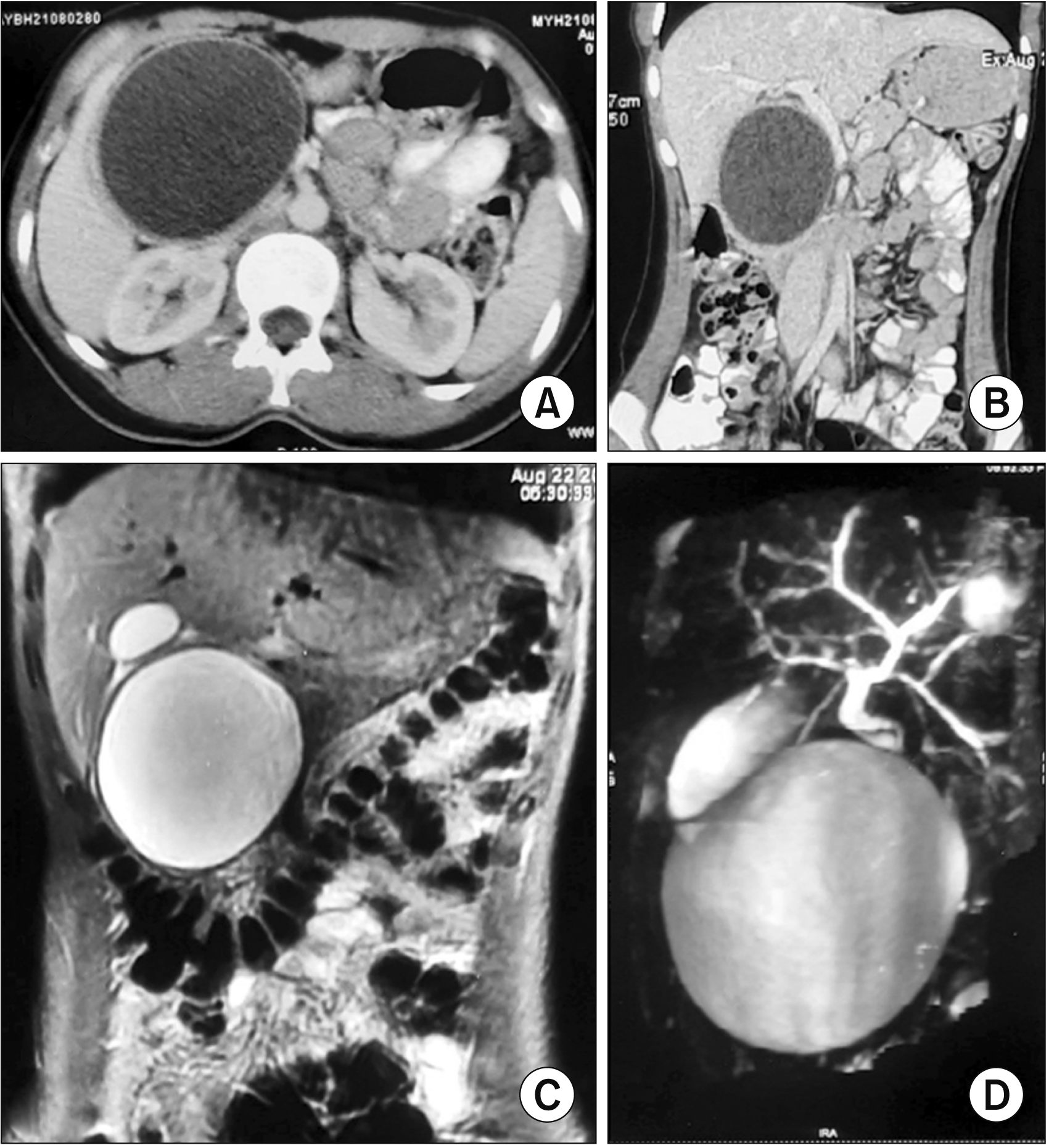 | Fig. 1(A) Coronal contrast-enhanced computed tomography (CT) image (axial view) showing a well-defined round unilocular cystic lesion in the head of the pancreas with a well-defined peripherally enhancing margin without any solid enhancing tissue or septations. (B) Contrast-enhanced CT image (coronal view) showing a well-defined round unilocular cystic lesion in the head of the pancreas with a well-defined peripherally enhancing margin without any internal solid component or septations. (C) Coronal T2W magnetic resonance imaging showing a well-defined smooth-walled unilocular cystic lesion in the head of the pancreas. (D) Magnetic resonance cholangiopancreatography image showing a large and well defined cystic lesion at the region of pancreatic head. The extrahepatic bile duct is seen separately without any evidence of bile duct dilatation. 
|
Since there was no significant improvement in symptoms in the next few months, the patient consulted at our center. The pancreatic cystic lesion was re-evaluated with repeat cross-sectional imaging. CECT of the abdomen showed a well-defined thin-walled hypodense cystic lesion (5.3 cm × 6.7 cm × 7.6 cm) with multiple, thin, linear, and wavy high attenuating structures suggesting floating membranes within it (water Lilly sign) in the pancreatic head and a thin rim of pancreatic parenchyma surrounding it (
Fig. 2A,
2B). MRI of the abdomen also showed similar findings (
Fig. 2C); hyperintense cystic lesion with multiple thin T2 hypointense floating membranes in the pancreatic head. The rest of the pancreas, including the main pancreatic duct, was found to be normal on both of these imaging. The biliary ductal system was found to be normal throughout its course on these imaging. There was no evidence of a similar cystic lesion in any other abdominal organ or in the chest. Based on these imaging findings, a diagnosis of primary cystic echinococcosis of the pancreas was suggested which was classified as CE3a stage (cyst with liquid contents and detached endocyst) as per the WHO-International Working Group on
Echinococcus (IWGE) classification [
6,
7].
 | Fig. 2(A) Plain computed tomography (CT) scan (axial view) showing a round well-defined cystic lesion in the head of the pancreas with multiple internal floating membranes. (B) Contrast-enhanced CT scan (axial view) showing a round well-defined hypodense cystic lesion in the head of the pancreas with multiple internal hyperdense floating membranes. (C) T2W magnetic resonance imaging image (axial view) showing the characteristic appearance of hydatid cyst as a well-defined cystic lesion with multiple floating membranes within (water Lilly sign). 
|
Routine hematological investigations, liver function test, and serum amylase (55 U/L) were within the normal range. Echinococcal serology was non-reactive (immunoglobulin [Ig] G 6 U/mL, normal range < 10 U/mL; IgM 10 U/mL, normal range < 20 U/mL).
After the diagnosis of primary isolated PHC, the patient underwent laparoscopic partial cystopericystectomy and omentoplasty with perioperative albendazole therapy (15 mg/kg/day). The laparoscopic procedure was performed using four ports (
Fig. 3) with the patient in the French position. Initial diagnostic laparoscopy revealed a large (~6 cm × 6 cm) thick wall cyst in the pancreatic head and uncinate process, splaying the duodenal C loop, bulging through the transverse mesocolon, and lying in-close approximation with the right and middle colic vessels (
Fig. 4A). The anterior aspect of the cyst was exposed by dividing the overlying gastrocolic omentum and mobilizing the proximal transverse colon (
Fig. 4B). The lesser sac was not entered at this stage to prevent its contamination with cyst contents during subsequent steps of the procedure. The cyst was isolated by placing 10% Povidone-iodine (scolicidal agent) soaked roller gauze around it (
Fig. 4B); cyst decompression was done with a laparoscopic aspiration needle (
Fig. 4C); scolicidal agent (10% Povidone-iodine solution) was injected into the cyst cavity with a sterilization time of 15 minutes; the contents aspirated again; the cyst was opened carefully at the most prominent point with an ultrasonic energy device (
Fig. 4D); cyst contents were evacuated with a 10 mm suction cannula (
Fig. 4E); cyst cavity was rinsed with scolicidal agent (10% Povidone-iodine) again; and then partial cystopericystectomy (deroofing) was done using ultrasonic shears (
Fig. 4F).
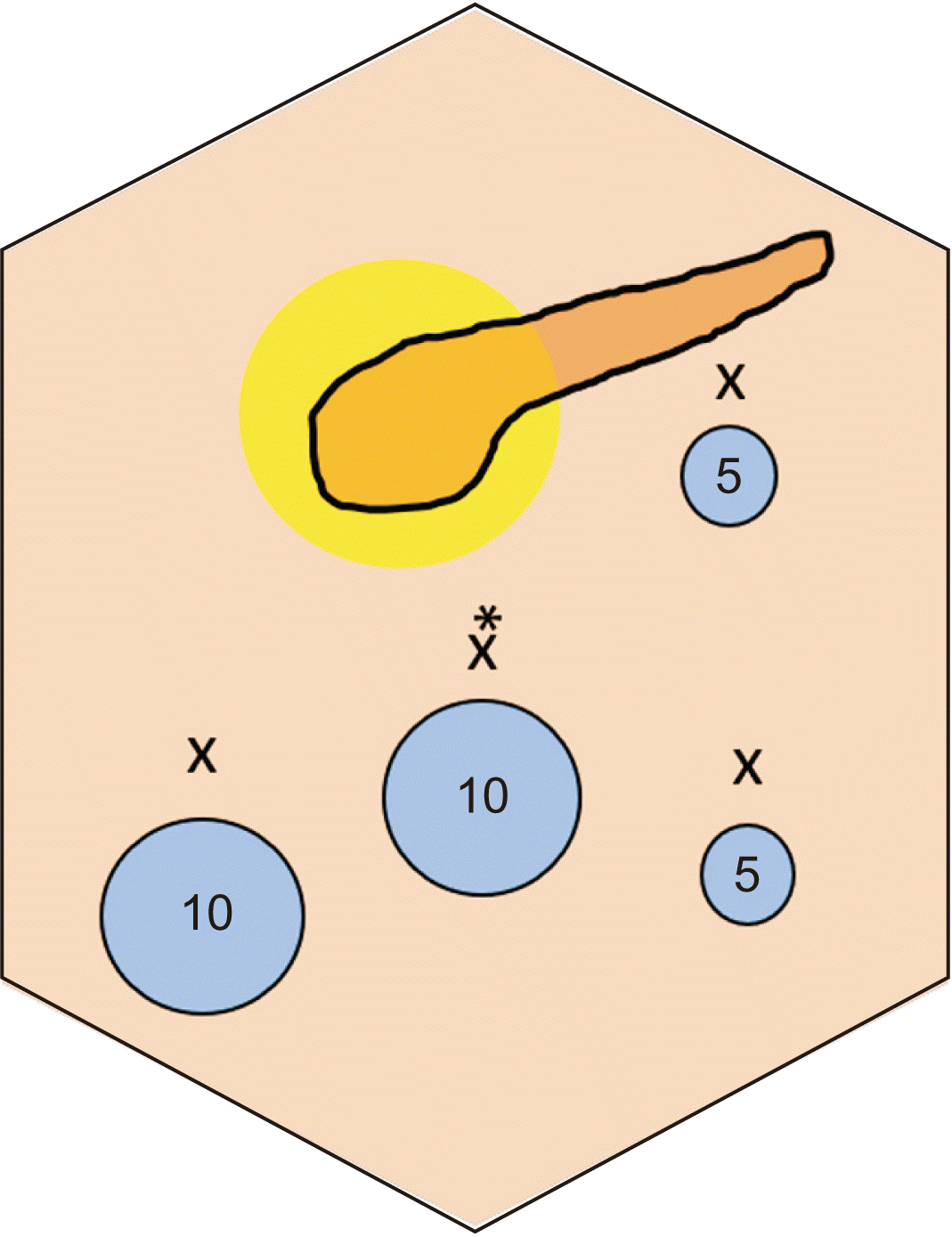 | Fig. 3Port positions (marked with 'X') used in the laparoscopic partial cystopericystectomy procedure. Two 10 mm ports (at the umbilicus [marked with *] for the camera, second for surgeon’s left working hand/suction/specimen extraction), two 5 mm ports (lower one for surgeon’s right working hand and upper one for assistant/surgeon). 
|
 | Fig. 4(A) Intraoperative image showing a large cystic lesion (*) in the head of the pancreas. (B) Cyst exposure after the division of overlying gastrocolic omentum. The cyst has been isolated from the surrounding structures by roller gauze soaked in scolicidal agent (10% Povidone-iodine). (C) Cyst content is being aspirated in a controlled manner. (D) Cyst is being opened with an ultrasonic energy device in a controlled manner with 2 suction cannulas placed close by to contain spillage. (E) Cyst contents (* membrane) are being evacuated with a 10 mm suction cannula. (F) Partial cystopericystectomy is being performed using ultrasonic energy device. (G) Opened up cyst after partial cystopericystectomy. (H) Omentoplasty. 
|
To prevent any spillage of cyst contents during the procedure, 2 suction cannulas were used during the initial opening and evacuation of the cyst cavity (
Fig. 4D) and one lip of the cyst wall (at the opening site) was retracted upwards throughout the cyst evacuation process (
Fig. 4E). Care was also taken during the “cystopericystectomy” step to protect adjoining luminal structures (e.g., duodenum, stomach, colon, pancreas) and vascular structures (e.g., right gastroepiploic vascular arcade, right colic, middle colic, and superior mesenteric vessels).
After partial cystopericystectomy, the cyst cavity was inspected for residual contents (
Fig. 4G), irrigated again with normal saline and 10% Povidone-iodine, omentoplasty was done (
Fig. 4H), and the cavity was drained with a closed suction drain (16F).
Excised cyst wall and membranes (
Fig. 5) were sent for histopathological examination that showed a lamellated membrane with an inner, degenerated germinal layer comprising degenerated protoscolices with hooklets and calcified areas, along with chronic pancreatitis changes in the adjoining pancreas (
Fig. 6). These findings confirmed the diagnosis of PHC.
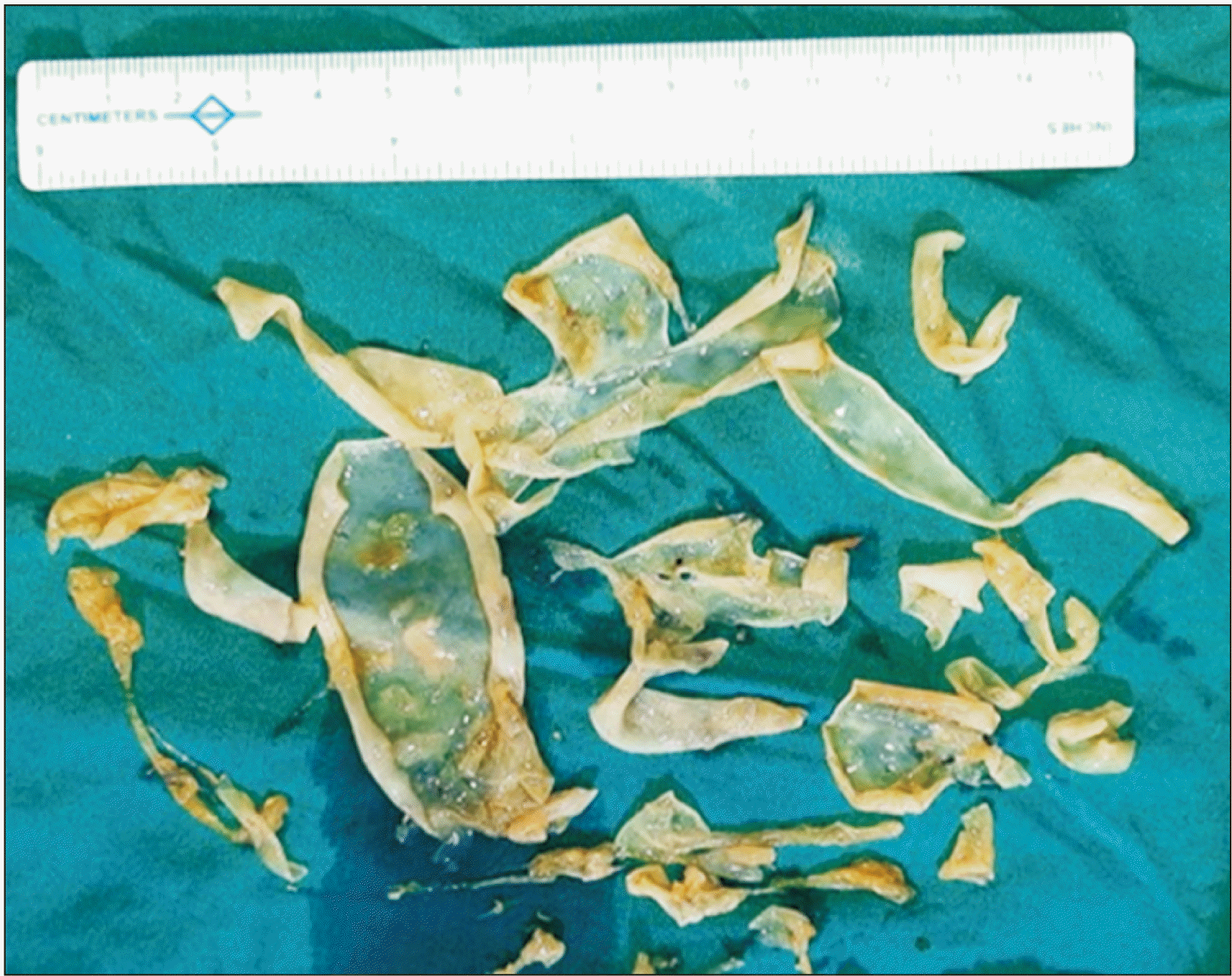 | Fig. 5Evacuated hydatid cyst membranes. 
|
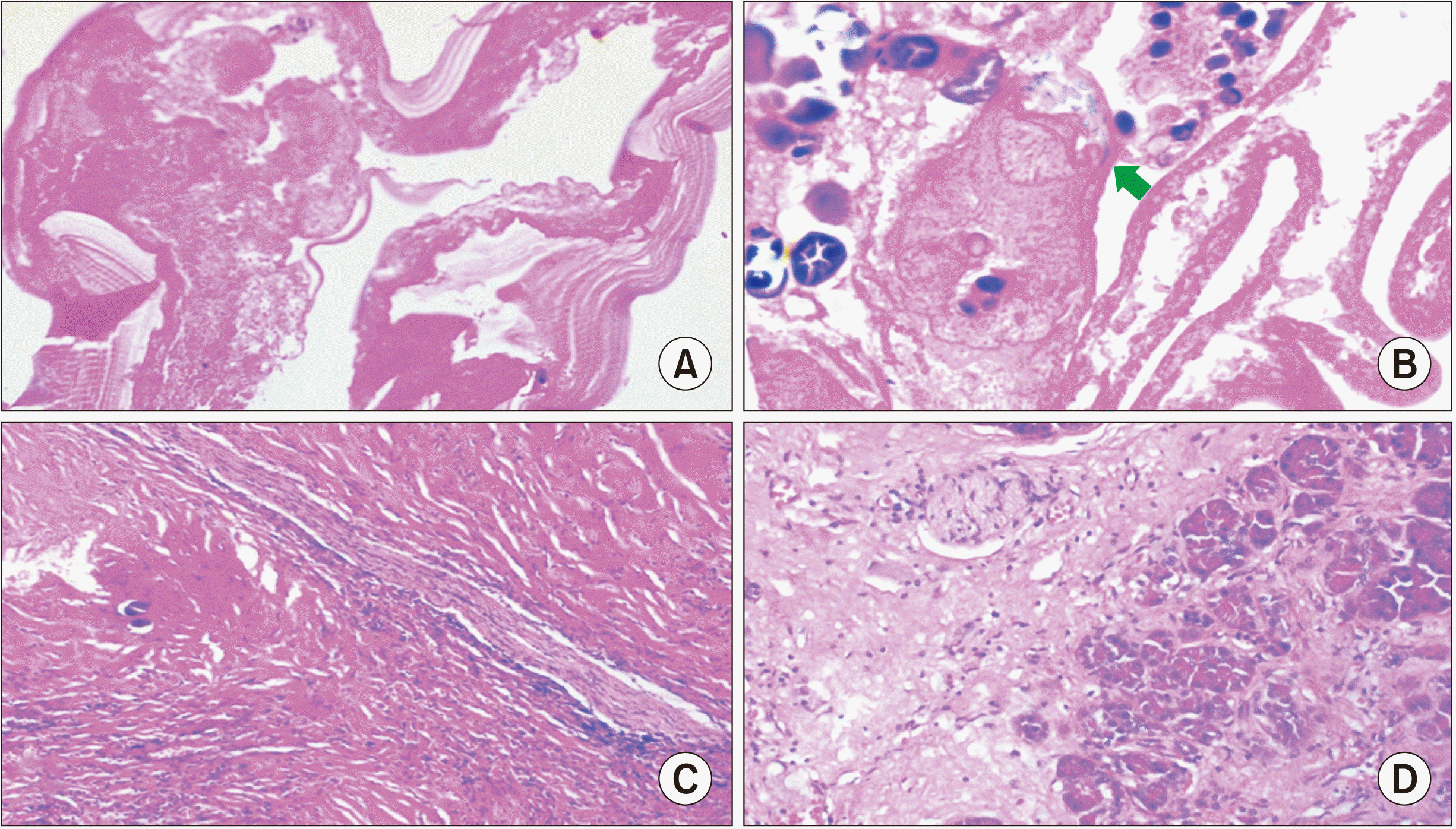 | Fig. 6(A) Hydatid cyst wall showing lamellated membrane with inner degenerative germinal layer (H&E, low power 200×). (B) The degenerative protoscolices of echinococcus with hooklets (arrow) (H&E, high power 400×). (C) Pericyst (H&E, low power 200×). (D) Adjoining pancreas showing chronic pancreatitis changes with acinar atrophy and interstitial fibrosis (H&E, low power 200×). 
|
There was a pancreatic leak in the postoperative period with a small fluid collection in the residual cavity that was managed conservatively. The patient was discharged on the 9th post-operative day on albendazole (15 mg/kg/day) for 8 weeks. The patient is doing well on follow-up at 3 months.
Go to :

DISCUSSION
Pancreatic involvement in hydatid disease is very rare with a reported incidence of 0.14% to 2% [
8]. Most of the aspects of primary PHC, including its natural history, diagnosis, and management, are not clear so far [
8]. The pancreas is thought to be involved primarily through hematogenous dissemination [
3]. Pancreatic hydatid involvement is solitary in a majority of cases [
1,
3]. It may remain asymptomatic and detected as an incidental finding [
9] or it may present with a variety of symptoms depending on its size, location, and associated complications. Common symptoms include epigastric/left upper quadrant pain and/or a lump, nausea, vomiting, and fever [
1].
Acute pancreatitis is a rare presentation that may occur due to pancreatic ductal obstruction by external compression by the cyst or by internal occlusion by scolices after cyst-ductal communication [
10]. Other rare complications include sinistral portal hypertension (by pancreatic tail lesions), rupture into the biliary tract, cholangitis, pancreatic fistula, and recurrent pancreatitis [
1]. Necrotising pancreatitis has also been reported [
10]. In the present case, abdominal pain was the primary symptom although the patient had acute pancreatitis (mild) initially. Associated chronic pancreatitis changes in the adjoining pancreas, as revealed on the histopathological examination and most probably due to chronic cyst compression, have not been reported so far.
Preoperative diagnosis of primary PHC is very difficult because symptoms are non-specific and characteristics radiological findings are not always present. As the cystic echinococcosis evolves, morphological changes in the cyst occur. Based on the morphological changes in imaging, cystic echinococcosis has been classified into five types or stages as per WHO-IWGE classification [
6,
7]. CL is a very initial or latent stage (unilocular cyst with no cyst wall or any pathognomonic signs). CE1 is an active stage with unilocular cyst having cyst wall and hydatid sand. CE2 is also an active stage with multivesicular cyst (rosette-like) with cyst wall. CE3 is a transitional cyst that has started to degenerate but may still produce a daughter cyst, thus it is a stage between an active cyst and inactive cyst; CE3a refers to a cyst with liquid contents and detached laminated membrane/endocyst and CEb refers to unilocular cyst with a predominantly solid component with daughter cysts. CE4 is an inactive phase and refers to a cyst with solid heterogenous degenerated contents without any daughter cyst. CE5, an inactive and the last stage, refers to a thick-walled cyst with partial or complete calcifications with degenerative contents [
6,
7].
Because of the wide variation in the imaging appearances, hydatid cysts can easily mimic other cystic lesions of the organ involved. PHC can mimic a choledochal cyst or pancreatic cystic neoplasm [
11,
12]. In fact, suspicion of the choledochal cyst was also raised during the initial evaluation in the present case. However, extrahepatic bile duct could be identified separately from the cyst and had normal appearance (
Fig. 1).
PHC can also mimic pancreatic pseudocyst. As the most common cystic lesion of the pancreas is pseudocyst, preoperative differentiation between a pancreatic pseudocyst and hydatid cyst is very important for appropriate management of the hydatid cyst and to avoid intraoperative and post-operative complications e.g., hypersensitivity reaction, spillage on the cyst contents with a risk of peritoneal hydatidosis. The presence of an isolated cystic lesion in the pancreas in association with an episode of acute pancreatitis requires a very high index of suspicion to diagnose cystic lesions other than a pancreatic pseudocyst. As hydatid cyst can also cause acute pancreatitis, unilocular hydatid cyst in its active early stages (i.e., CL, CE1 stages) can be very difficult to differentiate from a pseudocyst because of similar imaging features and very low index of suspicion as had been in the present case. However, as the hydatid cyst evolves, detached membranes or the development of a daughter cyst can help assess the true nature of the cystic lesion even in the context of acute pancreatitis as we have noted. In the present case, primary PHC evolved from CL/CE1 stage (
Fig. 1) to CE3a (
Fig. 2) over a period of time. Imaging re-evaluation after a few months showed classical features of hydatid cyst e.g., unilocular cyst with detached/floating membranes (water Lilly sign) (
Fig. 2) and helped in making the correct preoperative diagnosis.
CECT, MRI, or endoscopic ultrasonography are useful in the assessment of pancreatic cystic lesions. The presence of curvilinear calcification in the cyst wall, the presence of daughter cysts and hydatid sands, septations, or detached membranes can help distinguish hydatid cyst from more common cystic lesions (pseudocyst, cystic neoplasm) on imaging.
Serological testing for IgG and IgM may not be helpful as the seropositivity rate is lower for PHC (54%) than for liver hydatid disease [
1,
3]. Thus, negative serology does not rule out echinococcal disease as noted in the present case where the serology was negative.
Surgery is recommended for PHC to avoid subsequent complications [
13]. Medical treatment (albendazole) should be started at least 1 week prior to surgery and continued for 2 months postoperatively to avoid intraoperative anaphylaxis and to reduce postoperative recurrences.
Various surgical approaches have been described with a tendency toward conservative surgery for pancreatic head lesions and radical surgery for body and/or tail lesions [
13]. Internal drainage in the form of cystoenterostomy e.g., Roux-en-Y cystojejunostomy has been suggested to prevent postoperative pancreatic fistula or pseudocyst formation after a conservative surgical approach [
8]. Internal drainage is preferred in cases with suspected or confirmed communication with the main pancreatic duct (e.g., dilated pancreatic duct, recurrent episodes of acute pancreatitis). Pancreatic enzyme (amylase/lipase) assay in the initially aspirated cyst fluid and cysto-pancreatogram may be used, although with uncertain accuracy, in the intraoperative assessment of such cysto-pancreatic communication. In the present case, we resorted to conservative surgery (deroofing/partial cystopericystectomy) as the lesion was in the head of the pancreas although we did not perform internal drainage as the main pancreatic duct was normal with maintained fat planes with the cyst on preoperative imaging. Cyst fluid pancreatic enzyme assay or cysto-pancreatogram was not performed in the present case.
Radical surgery like pancreatoduodenectomy has been reported in patients with preoperative diagnostic uncertainty [
13]. Conventionally managed with an open approach [
13], there are very few reports of a laparoscopic approach for PHC which has been found to be feasible and safe [
8,
14-
16], but requires a surgical team with adequate experience in laparoscopic surgery to ensure intraoperative safe management of the cyst.
In conclusion, primary PHC is a rare entity that can masquerade as pseudocyst or cystic neoplasm, therefore, posing diagnostic and therapeutic challenges. Due to nonspecific symptoms, overlapping imaging features in the early stages and a low index of suspicion, correct preoperative diagnosis is rarely made. However, this entity should be kept in the differential diagnosis of a unilocular cystic lesion of the pancreas. A combination of imaging can help raise its suspicion or make a correct preoperative diagnosis. Surgery remains the most effective treatment modality. Perioperative chemotherapy is imperative to decrease the risk of recurrence. The laparoscopic approach is feasible and safe.
Go to :










 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download