Abstract
Splenic artery steal syndrome (SASS) is a cause of graft hypoperfusion leading to the development of biliary tract complications, graft failure, and in some cases to retransplantation. Its management is still controversial since there is no universal consensus about its prophylaxis and consequently treatment. We present a case of SASS that occurred 48 hours after orthotopic liver transplantation (OLTx) in a 56-year-old male patient with alcoholic cirrhosis and severe portal hypertension, and who was successfully treated by splenic artery embolization. A literature search was performed using the PubMed database, and a total of 22 studies including 4,789 patients who underwent OLTx were relevant to this review. A prophylactic treatment was performed in 260 cases (6.2%) through splenic artery ligation in 98 patients (37.7%) and splenic artery banding in 102 (39.2%). In the patients who did not receive prophylaxis, SASS occurred after OLTx in 266 (5.5%) and was mainly treated by splenic artery embolization (78.9%). Splenic artery ligation and splenectomies were performed, respectively, in 6 and 20 patients (2.3% and 7.5%). The higher rate of complications registered was represented by biliary tract complications (9.7% in patients who received prophylaxis and 11.6% in patients who developed SASS), portal vein thrombosis (respectively, 7.3% and 6.9%), splenectomy (4.8% and 20.9%), and death from sepsis (4.8% and 30.2%). Whenever possible, prevention is the best way to approach SASS, considering all the potential damage arising from an arterial graft hypoperfusion. Where clinical conditions do not permit prophylaxis, an accurate risk assessment and postoperative monitoring are mandatory.
Vascular complications are causes of graft failure in the early days after a liver transplantation, inducing hypoperfusion and ischemia, leading to biliary tract complications and occasionally retransplantation. One of the causes of arterial hypoperfusion of the graft in the absence of hepatic artery thrombosis (HAT) or hepatic artery stenosis (HAS) is the arterial steal syndrome, a nonocclusive hepatic artery hypoperfusion characterized by a blood-flow shift into other arteries originating from the same trunk and diagnosed on angiography. Most cases of liver ischemia are caused specifically by splenic artery steal syndrome (SASS), reported as a percentage ranging from 0.6% to 10% of patients [1].
SASS may develop any time, varying from the immediate postoperative period up to 5.5 years following transplantation, even though most patients are diagnosed within 2 months from the transplantation [1]. SASS is a diagnosis of exclusion and should be considered only in the absence of rejection, infection, or toxicity. Its clinical presentation is non-specific, ranging from a complete absence of symptoms to acute liver failure [1,2]. Most patients affected by this syndrome present with high levels of transaminases and decreased hepatic function with or without biliary ischemia and cholestasis [2,3], but sometimes they can be struck with ascites as the primary evidence of graft dysfunction and signs of hypersplenism [4,5]. Since the hypoperfusion may lead to the graft loss, not only is an early diagnosis extremely important, but also preoperative identification of patients at risk, followed, whenever possible, by prophylactic treatment.
The recipient was a 56-year-old male patient with alcoholic cirrhosis and severe portal hypertension, diagnosed with model for end-stage liver disease (MELD) 13, assessed according to our standard recipient protocol and placed on a transplantation list. Liver anatomy was evaluated through a computed tomography (CT) scan and magnetic resonance imaging with administration of Primovist, which showed features of chronic liver disease with portal hypertension, portal vein (PV) thrombosis extended to the superior mesenteric vein, and splenic and coronary veins dilatation. Hepatic artery anatomy was normal. A good-quality graft from a deceased donor was transplanted with face-to-face cavo-cavostomy, end-to-end portal vein, hepatic artery, and bile duct anastomosis. Intraoperative ultrasounds (IOUS) showed good intrahepatic inflow and outflow (index of resistence 0.60) and no sign of increased portal vein flow. At the end of the surgical procedure, blood testing showed lactates 5.2 mEq/L, lactate dehydrogenase (LDH) 3,503 mUI/L, glutamic-oxaloacetic transaminase (GOT) 1,542 UI/L, glutamic-pyruvic transaminase (GPT) 1,173 UI/L, total bilirubin 5.78 mg/dL, international normalized ratio (INR) 1.43, and fibrinogen 148 mg/dL.
The patient was transferred to the intensive care unit, intubated, with noradrenaline and dobutamine continuous infusion and started on immunosuppression through the combined administration of tacrolimus, mycophenolate mofetil, and steroid. Over the following 48 hours, the hepatic cytolysis enzymes did not show a downward trend, and lactates, INR, LDH, and serum bilirubin levels tended to rise (GOT 2,446 U/L, GPT 2,481 UI/L, LDH 1,940 mUI/L, lactates 6.3 mEq/L, total bilirubin 7.67 mg/dL, INR 2.37, fibrinogen 139 mg/dL). The patient, therefore, underwent a Doppler ultrasonography, which showed a great portal vein afflux (flow speed 44 cm/s) and no signal of intrahepatic arterial perfusion. A contrast-enhanced abdominal CT was performed (Fig. 1) in which a hepatic artery could be seen, but it seemed to be smaller; moreover, it supported the Doppler ultrasonography findings, showing no significant intrahepatic arterial perfusion. Consequently, the patient underwent angiography, confirming the presence of a very weak blood flow in the hepatic artery, completely shifted into the splenic artery, which showed an earlier filling (Fig. 2). Only after a selective access to the hepatic artery origin was it possible to correctly visualize the arterial anastomosis and the subsequent branches (Fig. 3), removing the suspicion of HAT.
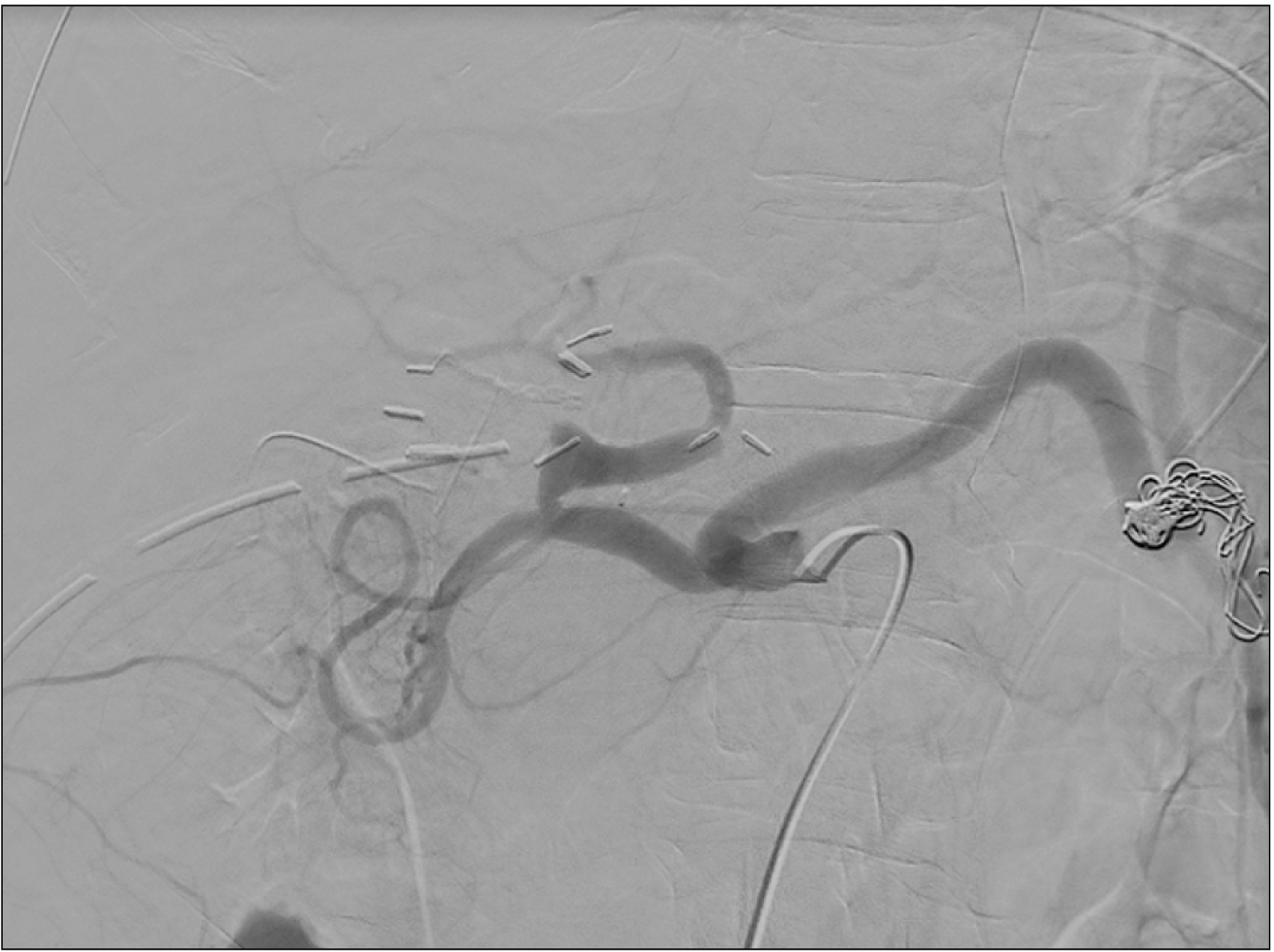
The treatment was a proximal splenic artery embolization, which allowed immediate restoration of hepatic artery flow (Fig. 4); moreover, the Doppler ultrasonography showed a normalization of portal flow speed (18 cm/s) and the presence of Doppler extra- and intrahepatic arterial signals.
The radiologic procedure was followed by a rapid decrease in cytolysis enzymes, total bilirubin, lactates, and INR (GOT 580 UI/L, GPT 1,554 UI/L, total bilirubin 6.40 mg/dL, lactates 2.1 mUI/L, INR 1.90), definitively normalized 5 days after the embolization.
Graft perfusion was controlled by Doppler ultrasound every 24 hours during the first 7 days after the procedure, and then every 72 hours until discharge. With the purpose of detecting the potential outbreak of biliary complications due to the ischemia, a control CT scan was performed both 3 and 15 days after embolization, showing only the presence of an ischemic area localized in the splenic inferior pole. The patient was discharged in good clinical condition at postoperative day 24. The follow-up at 3 and 6 months showed neither biliary tract complications nor splenic abscesses.
A literature search was performed using the PubMed database with the search key words splenic artery steal syndrome, splenic steal syndrome, arterial steal syndrome in orthotopic liver transplantation (OLTx), and nonocclusive hepatic artery hypoperfusion syndrome. We screened all titles, abstracts, and articles in the English language for review, carefully examining the data to remove double counting of patients between series; all the references of the articles found were reviewed to identify other studies that our research strategy might have missed. All the causes of graft hypoperfusion other than SASS were excluded from the analysis. Post-OLTx splenic artery steal syndrome was diagnosed by conventional angiography in all the patients included in this study.
A total of 22 studies including 4,789 patients who underwent OLTx were relevant to the prophylaxis or treatment of SASS (Table 1); among these, SASS occurred in a total of 266 patients.
A prophylactic treatment was performed in 260 cases (6.2%) through splenic artery embolization in 30 patients (11.5%), splenic artery ligation in 98 patients (37.7%), splenic artery banding in 102 patients (39.2%), and splenic artery temporary blockade in 28 patients (10.8%). One patient needed the arcuate ligament division (0.4%), and in one case an aortohepatic graft was required (0.4%).
SASS occurred after OLTx in 266 patients (5.5%, in line with the incidence reported in the literature reviewed) and was treated by splenic artery embolization in 210 patients (78.9%), splenic artery ligation in 6 patients (2.3%), splenic artery banding in 9 patients (3.4%); 2 patients were treated, respectively, through intra-arterial papaverine infusion and splenic artery narrowed stent (0.8%); 1 patient underwent revision of the hepatic artery anastomosis and gastroduodenal artery (GDA) coil embolization (0.4%); splenectomy was performed in 20 cases (7.5%); combined radiologic treatment, such as splenic artery and GDA or splenic artery and left gastric artery embolization, was performed in 2 patients (0.8%); during OLTx, 1 patient underwent intraoperative recipient common hepatic artery ligation after hypoperfusion over Carrel patch anastomosis (0.4%); in 1 patient an endoluminal hepatic artery stent was placed (0.4%). The treatment of the remaining 2 patients in whom SASS occurred is not available.
Thirteen (7.4%) patients with SASS received no treatment and were excluded from our analysis. Among these latter patients, impaired graft dysfunction occurred in 2 patients.
The higher rate of complications registered through the 2 groups of patients was represented by biliary tract complications (9.7% in patients who received prophylaxis and 11.6% in patients who developed splenic artery steal syndrome); PV thrombosis (respectively, 7.3% and 6.9%); the need for a secondary splenectomy (4.8% and 20.9%); death from sepsis (4.8% and 30.2%).
Both groups registered many cases of re-OLTx (a total of 24 patients, 12 among the cohort underwent prophylactic treatment and 12 who did not receive it); the main causes of retransplantation related to SASS were impaired graft dysfunction, portal vein thrombosis, hepatic artery thrombosis, and persistent biliary duct destruction).
All the other complications registered to the group of patients who underwent prophylactic treatment were 3 cases of postoperative bleeding (7.3%); biloma, which occurred in 2 patients (4.8%); and 1 case each of splenic artery thrombosis (2.4%) and splenic infarction (2.4%). Despite the prophylactic treatment, SASS occurred in 2 patients (4.8%) who underwent splenic artery banding, in 2 patients (4.8%) who underwent splenic artery ligation, and in 1 patient who underwent arcuate ligament division (1.2%).
In the group of patients who did not receive prophylaxis, the other complications registered after the treatment of SASS were 1 case of hepatic artery aneurysm (2.3%) and 2 cases of hepatic artery thrombosis (4.6%).
SASS is a controversial cause of nonocclusive hepatic arterial hypoperfusion in OLTx recipients caused by a true arterial “steal” from the hepatic artery towards the splenic artery, predominantly supported by angiography that demonstrates rapid and preferential filling of the splenic artery, and/or less commonly, of the GDA, instead of the hepatic artery. In 2008, Quintini et al. [2] proposed that the main cause of SASS was portal venous hyperperfusion, observing by Doppler ultrasonography an altered arterial blood supply to the liver associated with an increased portal venous flow. He suggested, therefore, 2 main mechanisms by which portal hyperperfusion caused sinusoidal injury in the graft: (1) elevated portal venous pressures (direct effect) and (2) hepatic artery hypoperfusion caused by the hepatic artery buffer response (HABR) related to rapid adenosine washout [1,2]. The adenosine works as an arterial vasodilator, and its rapid washout translates into hepatic artery vasoconstriction. Splenic artery embolization or ligation can prevent the steal from the graft by reducing the splenic vein contribution to the hepatic portal inflow, so that the portal vein hyperperfusion does not occur and the adenosine washout is reduced, maintaining its vasodilator effect on the hepatic artery.
The reason for portal venous hyperperfusion in posttransplant patients is not entirely clear; a discrepancy between the sizes of the transplant liver relative to the portal vein, as seen in undersized split grafts, has been implicated in portal venous hyperperfusion.
Since the graft hypoperfusion could lead to an early dysfunction and serious complications, mostly involving the bile duct and leading to graft loss, numerous efforts have been made to identify risk factors and stratify patients at risk to develop SASS. Some studies found that an enlarged splenic artery (> 4 mm or 150% of hepatic artery) or a difference between splenic and hepatic artery > 6 mm are accepted as risk factors of SASS [6,7], as well as a spleen volume > 829 mL assessed in the preoperative CT scan [5].
Angiography is essential in the diagnosis of SASS, and the typical diagnostic key point is a slow intra- and extrahepatic arterial flow relative to the splenic artery flow in the complete absence of an arterial anatomical defect such as HAS or thrombosis.
Currently, the available treatments, preventive, or curative, for SASS can vary from the interventional radiologic treatment (proximal coil embolization, Amplatzer vascular plug or intra-arterial papaverine infusion) to surgical ones, including splenic artery ligation or banding and splenectomy. Despite all these possibilities, most of the literature seems to prefer splenic artery proximal embolization for its minimally invasive approach, avoiding a surgical procedure that, in the case of intraoperative splenectomy or ligation of the splenic artery, could require an extended dissection area, increasing the risk of intra- and postoperative bleeding [1,3,5-20]. Splenic artery proximal embolization is defined as an embolization distal to the dorsal pancreatic artery (the first large branch) and proximal to the peripheral pancreatic magna artery (the second large branch). Moreover, the central placement of the coils in the splenic artery protects both the pancreatic inflow and the collateral blood supply to the spleen, preventing splenic infarction and abscesses, and reducing the infection rate, initially reported by Nüssler as up to 50% [3].
In the literature, Mogl et al. [7] reported 6 cases of splenectomy after embolization, without considering them as a complication of the treatment, and 6 cases of bile duct complications, suggesting the importance of a quick diagnosis to prevent long ischemia time; among these, 2 patients needed an hepaticojejunostomy and 4 underwent endoscopic retrograde choangiopancreatography.
With the same precaution of proximal embolization, splenic artery ligation seems to be a valid preventive alternative, especially in case of intraoperative evidence of SASS [3,7,11,21]. In the case reported by Rasmussen et al. [22], at the moment of the OLTx, an intraoperative ligation of the recipient common hepatic artery was performed since a Carrel patch was fashioned for the presence of an accessory right hepatic artery from the superior mesenteric artery.
To overcome the risk of splenic complications (such as infarctions or abscesses), the banding was introduced, allowing a decrease in splenic artery flow, with no irreversible influence and avoiding local ischemic necrosis in the spleen [3,7]. However, in the series reported by Mogl et al. [7], 2 patients developed SASS after banding, suggesting that even if it can modulate the blood flow, it might not prevent this syndrome, requiring an ulterior approach.
Splenectomy could be an effective therapeutic option, but current recommendations, considering the high risk of this procedure in patients with severe portal hypertension, limit it to cases of additional pathology, such as an aneurysm of the splenic artery [3,23].
The intra-arterial infusion of the vasodilatory drug (papaverine) was performed in only one case, reported by Kirbas et al. [6]. Even if it is widely accepted in the management of nonocclusive mesenteric ischemia, there were no other authors who used this approach in the management of SASS, so that to analyze results from this conservative treatment, further studies might be necessary. Although after liver transplantation, the vasoconstriction leading to steal syndrome is due to a rapid adenosine washout caused by the increased portal inflow, the intra-arterial perfusion of a vasodilatory drug could be really effective to reverse the vasoconstriction and modulate the HABR, but it would not solve the problem of portal circulation imbalance.
Many of the improvements in the management and prevention of SASS come from the growing experience in living-donor liver transplantation (LDLT), in which the phenomenon of small-for-size syndrome has reached attention. A prospective study by Troisi et al. [24] showed that the modulation of recipient portal inflow through ligation or embolization of the splenic artery could improve liver function and increase hepatic artery inflow, avoiding portal hyperperfusion and small-for-size syndrome. The sharing of the same pathophysiologic pattern involving the hepatic artery buffer response seems to explain the successful treatment of SASS by splenic artery inflow modulation. In the study conducted by Umeda et al. [8] in the LDLT setting, preoperative splenic artery embolization (12–18 hours before surgery) in patients with severe portal hypertension proved to prevent graft hypoperfusion and ensured shorter operative times and less blood loss.
In the review conducted, only 5 authors reported prophylaxis of SASS [3,7,8,21,25], and all the evidence deriving from the literature seems to suggest that although the prophylaxis is not exempt from risks and complications (such as postoperative bleeding, splenectomy, sepsis, as well as the occurrence of SASS), these latter can carry less risk than those deriving from the steal syndrome and prolonged graft hypoperfusion. Moreover, SASS is in the framework of time-dependent pathologies since its impact on the graft, which in the most extreme cases can lead to re-transplantation, is closely related to the ischemia time. The clinical presentation is not always obvious, and the wide range of differential diagnosis imposes an accurate risk assessment of all patients who are candidates for OLTx.
In conclusion, we believe that, whenever possible, prevention is the best way to approach SASS, considering all the damages potentially arising from arterial graft hypoperfusion. However, PV thrombosis, or the presence of a transhepatic intrajugular porto-systemic shunt or other porto-caval shunts are contraindication for prophylactic treatment since these patients seem to be at higher risk of developing PV thrombosis after OLTx (5%–21%) or worsening a pre-existing PV thrombosis [26]. In this context, the best clinical practice is a careful stratification of the patients at risk of developing a SASS and a meticulous monitoring by blood test and Doppler ultrasound to precociously detect indirect signs of hypoperfusion and quickly access angiography.
REFERENCES
1. Uflacker R, Selby JB, Chavin K, Rogers J, Baliga P. 2002; Transcatheter splenic artery occlusion for treatment of splenic artery steal syndrome after orthotopic liver transplantation. Cardiovasc Intervent Radiol. 25:300–306. DOI: 10.1007/s00270-002-2614-5. PMID: 12042994.
2. Quintini C, Hirose K, Hashimoto K, Diago T, Aucejo F, Eghtesad B, et al. 2008; "Splenic artery steal syndrome" is a misnomer: the cause is portal hyperperfusion, not arterial siphon. Liver Transpl. 14:374–379. DOI: 10.1002/lt.21386. PMID: 18306381.
3. Nüssler NC, Settmacher U, Haase R, Stange B, Heise M, Neuhaus P. 2003; Diagnosis and treatment of arterial steal syndromes in liver transplant recipients. Liver Transpl. 9:596–602. DOI: 10.1053/jlts.2003.50080. PMID: 12783401.
4. Geissler I, Lamesch P, Witzigmann H, Jost U, Hauss J, Fangmann J. 2002; Splenohepatic arterial steal syndrome in liver transplantation: clinical features and management. Transpl Int. 15:139–141. DOI: 10.1111/j.1432-2277.2002.tb00141.x. PMID: 11935171.
5. Grieser C, Denecke T, Steffen IG, Avgenaki M, Fröhling V, Mogl M, et al. 2010; Multidetector computed tomography for preoperative assessment of hepatic vasculature and prediction of splenic artery steal syndrome in patients with liver cirrhosis before transplantation. Eur Radiol. 20:108–117. DOI: 10.1007/s00330-009-1535-y. PMID: 19662418.
6. Kirbas I, Ulu EM, Ozturk A, Coskun M, Harman A, Ogus E, et al. 2007; Multidetector computed tomographic angiography findings of splenic artery steal syndrome in liver transplantation. Transplant Proc. 39:1178–1180. DOI: 10.1016/j.transproceed.2007.02.024. PMID: 17524925.
7. Mogl MT, Nüssler NC, Presser SJ, Podrabsky P, Denecke T, Grieser C, et al. 2010; Evolving experience with prevention and treatment of splenic artery syndrome after orthotopic liver transplantation. Transpl Int. 23:831–841. DOI: 10.1111/j.1432-2277.2010.01062.x. PMID: 20180930.
8. Umeda Y, Yagi T, Sadamori H, Matsukawa H, Matsuda H, Shinoura S, et al. 2007; Preoperative proximal splenic artery embolization: a safe and efficacious portal decompression technique that improves the outcome of live donor liver transplantation. Transpl Int. 20:947–955. DOI: 10.1111/j.1432-2277.2007.00513.x. PMID: 17617180.
9. Uslu N, Aslan H, Tore HG, Moray G, Karakayali H, Boyvat F, et al. 2012; Doppler ultrasonography findings of splenic arterial steal syndrome after liver transplant. Exp Clin Transplant. 10:363–367. DOI: 10.6002/ect.2012.0007. PMID: 22757943.
10. Zhu X, Tam MD, Pierce G, McLennan G, Sands MJ, Lieber MS, et al. 2011; Utility of the Amplatzer Vascular Plug in splenic artery embolization: a comparison study with conventional coil technique. Cardiovasc Intervent Radiol. 34:522–531. DOI: 10.1007/s00270-010-9957-0. PMID: 20700592.
11. García-Criado A, Gilabert R, Bianchi L, Vilana R, Burrel M, Barrufet M, et al. 2015; Impact of contrast-enhanced ultrasound in the study of hepatic artery hypoperfusion shortly after liver transplantation: contribution to the diagnosis of artery steal syndrome. Eur Radiol. 25:196–202. DOI: 10.1007/s00330-014-3377-5. PMID: 25117745.
12. Shimizu K, Tashiro H, Fudaba Y, Itamoto T, Ohdan H, Fukuda S, et al. 2007; Splenic artery steal syndrome in living donor liver transplantation: a case report. Transplant Proc. 39:3519–3522. DOI: 10.1016/j.transproceed.2007.06.074. PMID: 18089426.
13. Chao CP, Nguyen JH, Paz-Fumagalli R, Dougherty MK, Stockland AH. 2007; Splenic embolization in liver transplant recipients: early outcomes. Transplant Proc. 39:3194–3198. DOI: 10.1016/j.transproceed.2007.07.089. PMID: 18089351.
14. Lima CX, Mandil A, Ulhoa AC, Lima AS. 2009; Splenic artery steal syndrome after liver transplantation: an alternative technique of embolization. Transplant Proc. 41:1990–1993. DOI: 10.1016/j.transproceed.2009.01.086. PMID: 19545776.
15. Kim JH, Kim KW, Gwon DI, Ko GY, Sung KB, Lee J, et al. 2011; Effect of splenic artery embolization for splenic artery steal syndrome in liver transplant recipients: estimation at computed tomography based on changes in caliber of related arteries. Transplant Proc. 43:1790–1793. DOI: 10.1016/j.transproceed.2011.02.022. PMID: 21693280.
16. Sevmis S, Boyvat F, Aytekin C, Gorur SK, Karakayali H, Moray G, et al. 2006; Arterial steal syndrome after orthotopic liver transplantation. Transplant Proc. 38:3651–3655. DOI: 10.1016/j.transproceed.2006.10.145. PMID: 17175358.
17. Zhu XS, Gao YH, Wang SS, Cheng Q, Ling Y, Fan L, et al. 2012; Contrast-enhanced ultrasound diagnosis of splenic artery steal syndrome after orthotopic liver transplantation. Liver Transpl. 18:966–971. DOI: 10.1002/lt.23453. PMID: 22511324.
18. Li C, Quintini C, Hashimoto K, Fung J, Obuchowski NA, Sands MJ, et al. 2016; Role of Doppler sonography in early detection of splenic steal syndrome. J Ultrasound Med. 35:1393–1400. DOI: 10.7863/ultra.15.06072. PMID: 27208197.
19. Liu DY, Yi ZJ, Tang Y, Niu NN, Li JX. 2015; Three case reports of splenic artery steal syndrome after liver transplantation. Transplant Proc. 47:2939–2943. DOI: 10.1016/j.transproceed.2015.10.037. PMID: 26707318.
20. Pérez J, Grande LG, Achécar L, Moreno A, Fortún J, Arranz G, et al. 2011; Case report of splenic artery steal syndrome: demonstration of portal hyperflow mechanism by anatomic variant of the splenic artery and correlation with Doppler rates. Transplant Proc. 43:2269–2271. DOI: 10.1016/j.transproceed.2011.05.022. PMID: 21839253.
21. Wojcicki M, Pakosz-Golanowska M, Lubikowski J, Post M, Jarosz K, Milkiewicz P. 2012; Direct pressure measurement in the hepatic artery during liver transplantation: can it prevent the "steal" syndrome? Clin Transplant. 26:223–228. DOI: 10.1111/j.1399-0012.2011.01478.x. PMID: 21554400.
22. Rasmussen A, Hjortrup A, Kirkegaard P. 1997; Intraoperative measurement of graft blood flow--a necessity in liver transplantation. Transpl Int. 10:74–77. DOI: 10.1111/j.1432-2277.1997.tb00541.x. PMID: 9002157.
23. Sainz-Barriga M, Baccarani U, Risaliti A, Gasparini D, Sponza M, Adani GL, et al. 2004; Successful minimally invasive management of late portal vein thrombosis after splenectomy due to splenic artery steal syndrome following liver transplantation: a case report. Transplant Proc. 36:558–559. DOI: 10.1016/j.transproceed.2004.02.040. PMID: 15110593.
24. Troisi R, Cammu G, Militerno G, De Baerdemaeker L, Decruyenaere J, Hoste E, et al. 2003; Modulation of portal graft inflow: a necessity in adult living-donor liver transplantation? Ann Surg. 237:429–436. DOI: 10.1097/01.SLA.0000055277.78876.B7. PMID: 12616129. PMCID: PMC1514313.
25. Song JY, Shi BY, Zhu ZD, Zheng DH, Li G, Feng LK, et al. 2014; New strategies for prevention and treatment of splenic artery steal syndrome after liver transplantation. World J Gastroenterol. 20:15367–15373. DOI: 10.3748/wjg.v20.i41.15367. PMID: 25386086. PMCID: PMC4223271.
26. Manzanet G, Sanjuán F, Orbis P, López R, Moya A, Juan M, et al. 2001; Liver transplantation in patients with portal vein thrombosis. Liver Transpl. 7:125–131. DOI: 10.1053/jlts.2001.21295. PMID: 11172396.
Fig. 1
The contrast-enhanced abdominal computed tomography performed between Doppler ultrasounds and angiography.
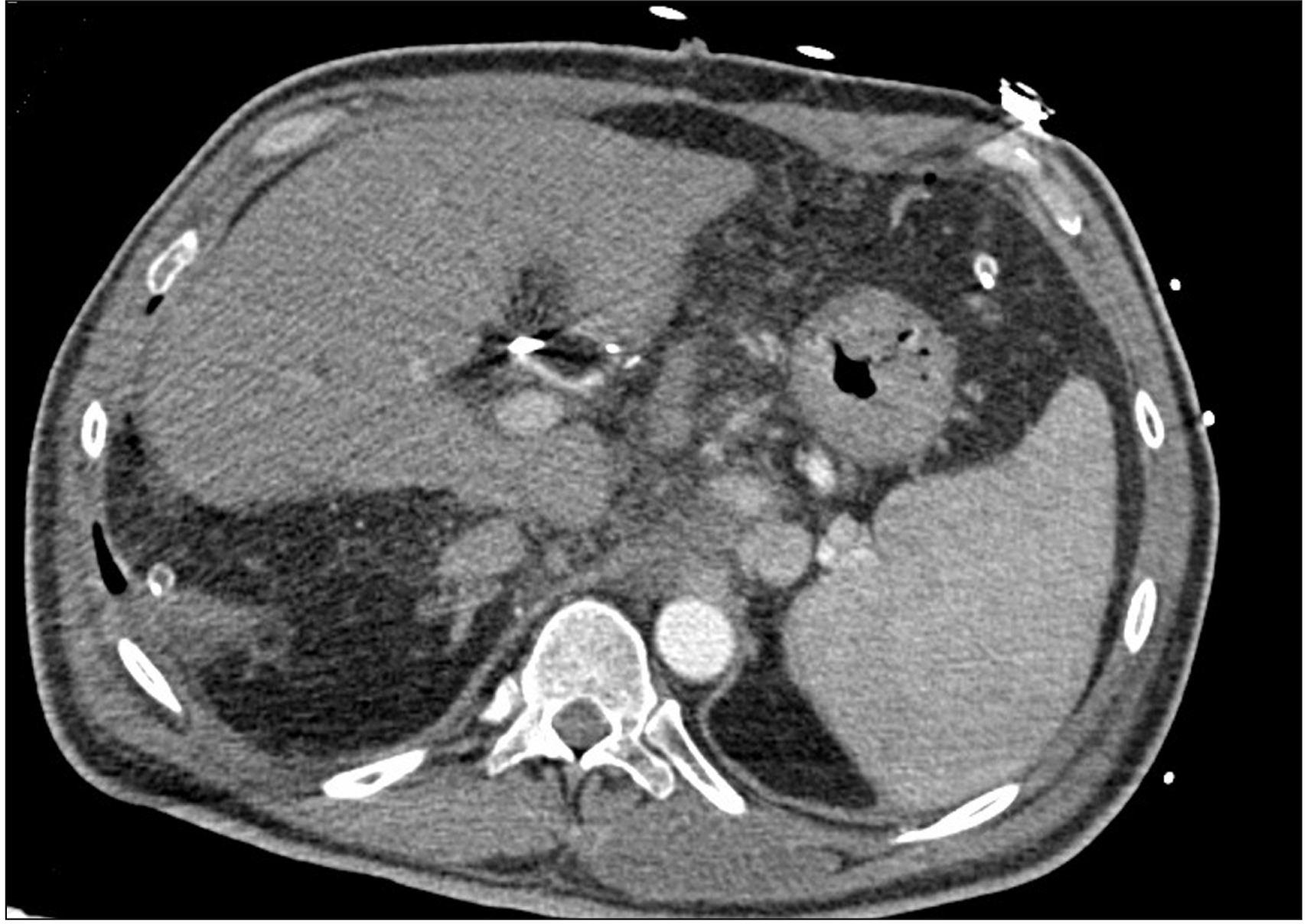
Fig. 2
Angiography before splenic artery embolization. A diversion of blood flow into the splenic artery is shown, and the hepatic artery is not correctly visualized.
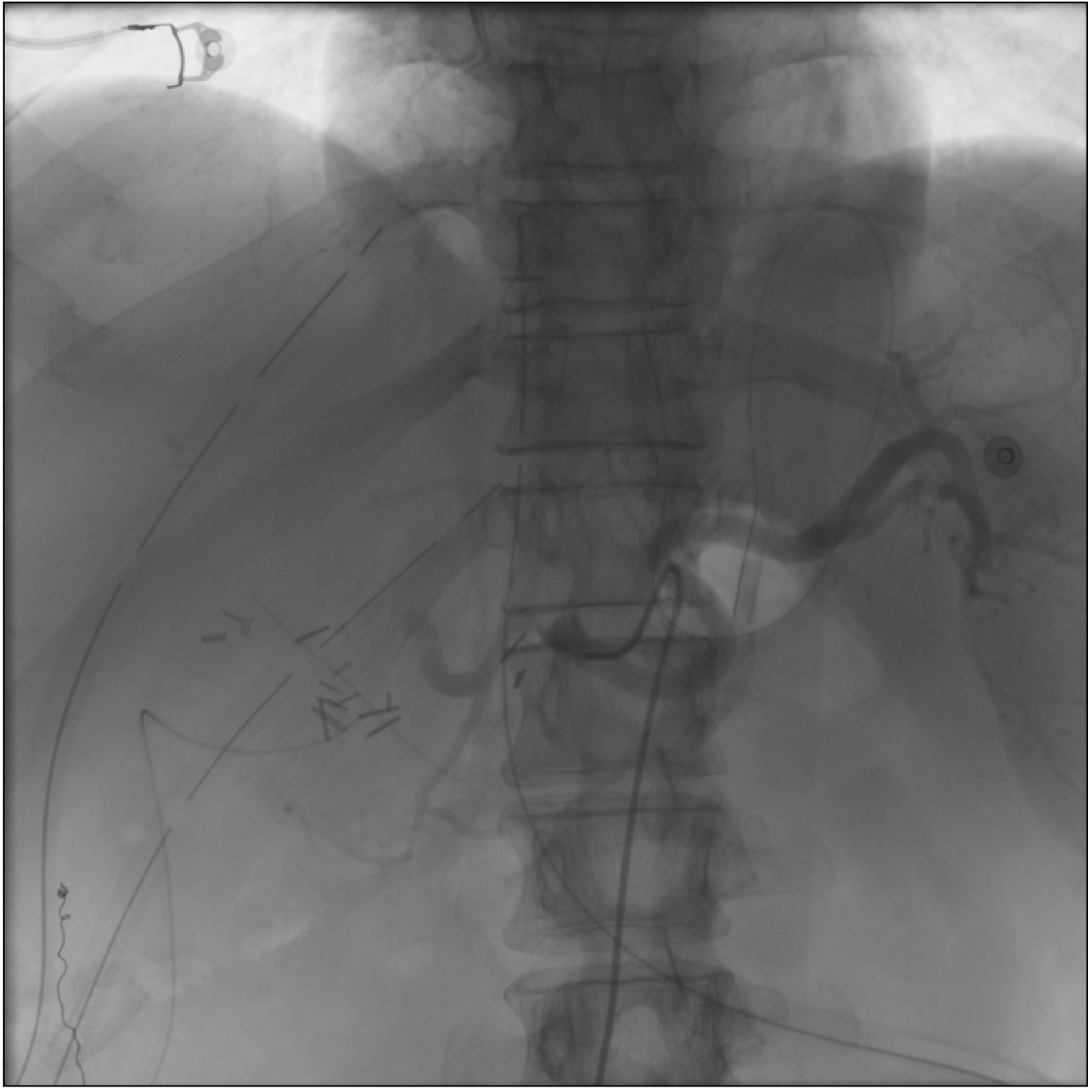
Fig. 3
Angiography before splenic artery embolization. Selective access to the hepatic artery origin, visualizing arterial anastomosis and the subsequent branches.
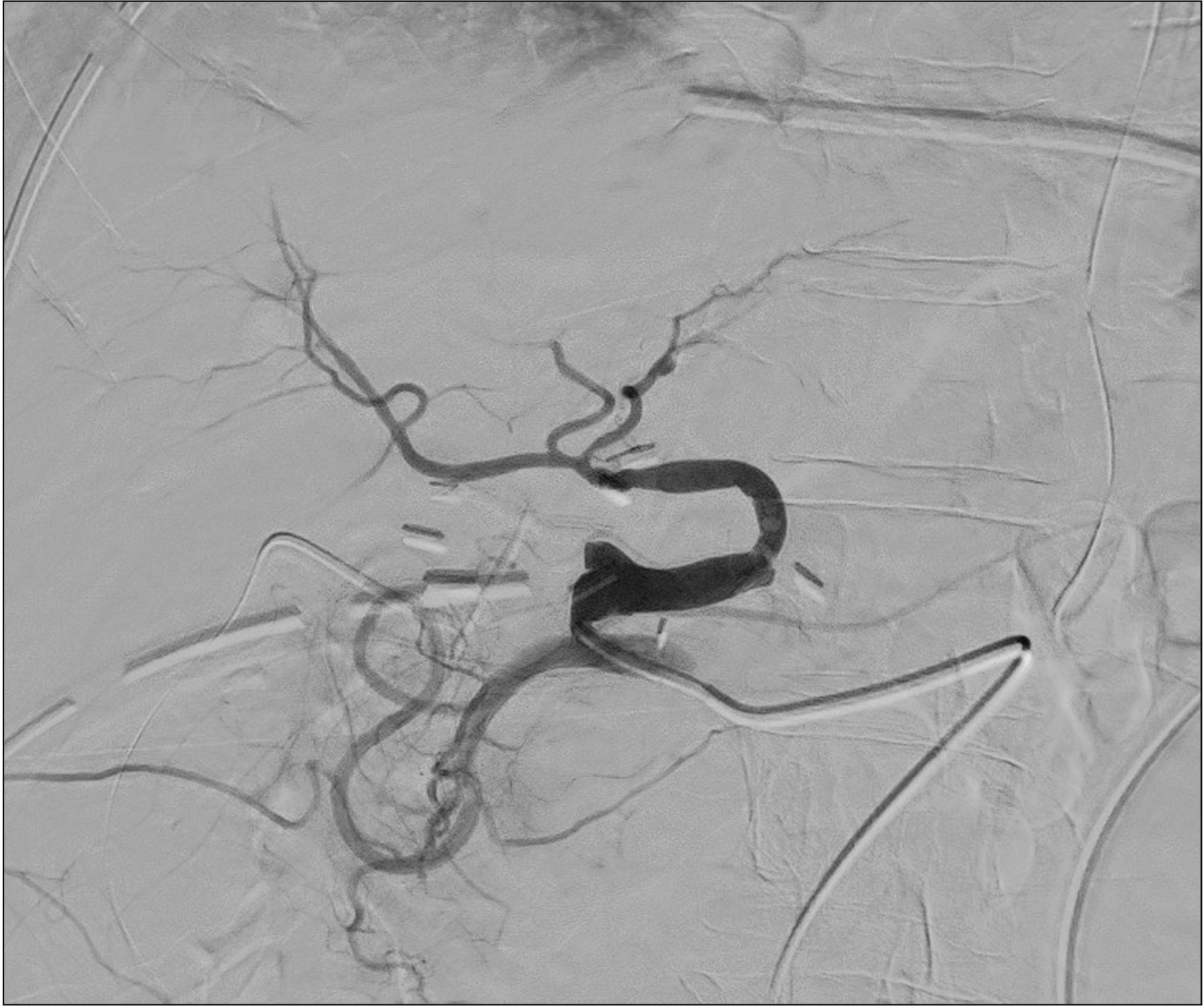
Table 1
Main features of all the studies considered for the literature review
| Author | Journal | Type of article | Patient (n) | SASS (n) | Prophylaxis or treatment (n) | Post treatment event (n) | Outcome |
|---|---|---|---|---|---|---|---|
| Umeda et al. 2007 [8] | Transplant international | Randomized clinical trial | 60 | 2 |
Prophylaxis (30) • Splenic artery embolization (30) |
• Postoperative bleeding (2) • Biloma (2) • Acute rejection (9) |
Decreased blood loss, short operation time, lower mortality rate |
|
Treatment (2) • Splenic artery embolization (2) |
• Postoperative bleeding (6) • Biloma (3) • Acute rejection (5) |
||||||
| Wojcicki et al. 2012 [21] | Clinical transplantation | Journal article | 99 | 1 |
Prophylaxis (7) • Splenic artery ligation (5) • Arcuate ligament division (1) • Aortohepatic bypass grafting (1) |
• SASS (1)a) |
Normalization of arterial inflow pressure in the graft |
| Mogl et al. 2010 [7] | Transplant international | Original article | 1,008 | 27 |
Prophylaxis (98) • Splenic artery banding (20) |
• SASS (2)b) • Splenectomy (1) • Biliary tract complications requiring hepaticojejunostomy (3) |
Lower rate of complication after prophylaxis compared to the treatment of SASS |
| • Splenic artery ligation (78) |
• SASS (2)c) • Bleeding after splenic artery ligation requiring reintervention (1) • Splenic infarction (1) • Portal vein thrombosis (2) • Re-OLTx (12) |
||||||
|
Treatment (23) • Splenic artery embolization (23) |
• Biliary tract complication requiring hepaticojejunostomy (2) • Biliary tract complication requiring ERCP (3) • Hepatic artery aneurysm (1) • Hepatic artery thrombosis (1) • Splenectomy (1) • Re-OLTx (1) • Death for sepsis and multiorgan failure (1) |
||||||
| Song et al. 2014 [25] | World Journal of Gastroenterology | Clinical trials study | 43 | 0 |
Prophylaxis (28) • Splenic artery temporary blockade (28) |
• Splenic artery thrombosis (1) • Biliary tract complications (1) |
Temporary blockade of the splenic artery can prevent SASS and all the complication related to the artery ligation, such as local ischemic necrosis of the spleen |
| Grieser et al. 2010 [5] | European Radiology | Journal article | 119 | 12 |
Treatment (12) • Splenic artery embolization) |
Not available | Not available |
| Uflacker et al. 2002 [1] | CardioVascular and Interventional Radiology | Journal article | 350 | 11 |
Treatment (11) • Splenic artery embolization) |
• Hepatic artery thrombosis (1) |
Embolization is a minimally invasive treatment, leading to immediate clinical improvement |
| Kirbas et al. 2007 [6] | Transplantation Proceeding | Journal article | 198 | 8 |
Treatment (8) • Partial splenic artery embolization (6) • Splenic artery narrowed stent (1) • Intra-arterial papaverine infusion (1) |
Not available | Not available |
| Nüssler et al. 2003 [3] | Liver transplantation | Original article | 1,171 | 69 |
Prophylaxis (97) • Splenic artery ligation (15) • Splenic artery banding (82) • Treatment (69) • Splenic artery embolization (29) • Splenic artery banding (9) • Splenectomy (18) No treatment (13) |
• Splenectomy (1) • Death for sepsis (1) • Portal vein thrombosis (1) • Death for sepsis (1) • Portal vein thrombosis (3) • Splenectomy (8) • Septic complication (12) • Re-OLTx (7) • No complications • Re-OLTx (2) • Impaired graft function (2) |
Splenic artery ligation has greater morbidity than banding, but both can prevent SASS |
| Uslu et al. 2012 [9] | Experimental and clinical transplantation | Journal article | 210 | 20 |
Treatment (20) • Splenic artery embolization (20) |
Not reported | Not reported |
| Zhu et al. 2011 [10] | CardioVascular and Interventional Radiology | Comparative study | 247 | 23 |
Treatment (23) • Splenic artery embolization (23) |
Not reported | Not reported |
| García-Criado et al. 2015 [11] | European Radiology | Journal article | 675 | 7 |
Treatment (7) • Splenic artery ligation (5) • Splenic artery embolization (2) |
Not reported | Normalization of intrahepatic arterial flow after 24 hours from the treatment |
| Sainz-Barriga et al. 2004 [23] | Transplantation proceedings | Case report | 1 | 1 |
Treatment (1) • Partial occlusion of splenic artery and splenectomy |
• Portal vein thrombosis (1) treated by heparin infusion and percutaneous thrombectomy and rTPA infusion |
Normalization of intrahepatic arterial flow after the treatment, good clinical condition at 6 months follow-up |
| Shimizu et al. 2007 [12] | Transplantation proceedings | Case report | 1 | 1 |
Treatment (1) • Splenic artery embolization |
None after embolization | Normalization of intrahepatic arterial flow and liver function improvement immediately after the treatment; good clinical condition at 6 months follow-up |
| Chao et al. 2007 [13] | Transplantation proceedings | Outcome report | 5 | 1 |
Treatment (1) • Splenic artery embolization |
Mild transient pulmonary oedema, likely unrelated to the embolization, treated by diuretics | Normalization of intrahepatic arterial flow; resolution of the ascites within 2 weeks; good clinical condition at 43 months follow-up |
| Lima et al. 2009 [14] | Transplantation proceedings | Case report | 1 | 1 |
Treatment (1) • Splenic artery embolization |
None after embolization | Normalization of intrahepatic arterial flow; good clinical condition at 13 months follow-up |
| Kim et al. 2011 [15] | Transplantation proceedings | Case report | 73 | 9 |
Treatment (9) • Splenic artery embolization |
• Subclinical minor splenic infarction (2) • Minor biliary complication (1) |
Normalization of intrahepatic arterial flow; no evidence of rejection or major vascular complications at 12 months follow-up |
| Rasmussen et al. 1997 [22] | Transplant international | Case report | 70 | 1 |
Treatment (1) • Intraoperative recipient common hepatic artery ligation at the moment of OLTx |
None after ligation | Normalization of the arterial graft flow |
| Sevmis et al. 2006 [16] | Transplantation proceedings | Original article | 118 |
10 • SASS (9) • SASS + LGASS (1) |
Treatment (10) • Splenic artery embolization (8) • Endoluminal stent placement (1) • Splenic artery and left gastric artery embolization (1) |
• Acute rejection (5) • Re-OLTx for chronic rejection (1) |
Normalization of the intrahepatic arterial flow; median follow-up 10.2 months |
| Zhu et al. 2012 [17] | Liver transplantation | Original article | 236 | 8 |
Treatment (8) • Splenic artery embolization |
Not reported | Normalization of the intrahepatic arterial flow |
| Li et al. 2016 [18] | Journal of ultrasound in medicine | Original research | 100 | 50 |
Treatment (50) • Splenic artery embolization |
• Death (5, multiple organ failure, acute liver failure, graft failure due to hepatic artery thrombosis) • Re-OLTx (1) |
Normalization of the intrahepatic arterial flow |
| Liu et al. 2015 [19] | Transplantation proceedings | Case report | 3 | 3 |
Treatment (3) • Splenic artery embolization (3) |
In 1 patient hepatic artery inflow was not immediately restored after splenic artery embolization, but required some days | Progressive normalization of the intrahepatic arterial flow |
| Pérez et al. 2011 [20] | Transplantation proceedings | Case report | 1 | 1 |
Treatment (1) • GDA embolization and partial splenic artery embolization |
Chronic rejection | Immediate improvement of the intrahepatic arterial flow; good clinical condition at 12 months follow-up |
| Total (n = 22) | 4,789 | 266 |
Prophylaxiys → 260 Treatment → 266 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download