INTRODUCTION
Pancreatic ductal disruptions are known to occur secondary to acute pancreatitis, chronic pancreatitis, abdominal trauma, post-surgery and malignancies [
1]. They can manifest in the form of internal pancreatic fistulae (IPF) presenting with pancreatic ascites, pleural effusion, or large peri-pancreatic collections. They can also manifest in the form of external pancreatic fistulae (EPF) in post-surgical settings and after percutaneous drainage of peri-pancreatic collections. Ductal disruption may involve the main pancreatic duct or side branches. Clinical sequelae that develop subsequent to a pancreatic leak are dependent on etiology, site of leak, extent of leak, local inflammatory reaction, and pancreatic ductal anatomy with presence of calculi and strictures [
2]. Anterior disruptions in the pancreatic duct usually lead to leakage of pancreatic fluid into the peritoneum with pancreatic ascites. On the other hand, posterior disruptions can track into the mediastinum and lead to pancreatic pleural effusions [
1]. External fistula usually occurs as a consequence of percutaneous drainage of pancreatic fluid collections [
3]. Fistulae can be classified as low output (< 200 mL/day) or high output (> 200 mL/day) [
4]. Therapy in pancreatic fistula is complex as compared to biliary leaks. Due to high pressure in the pancreatic duct with tonic and phasic secretory regulation, sphincterotomy alone to reduce pressure gradient may not heal the fistula [
5]. Endoscopic transpapillary stent placement along with sphincterotomy is usually undertaken as the first therapeutic strategy for patients with IPF or EPF, especially when the fistula output is high. Clinical success of endoscopic stent placement ranges from 55% to 100% in IPF and from 80% to 100% in EPF [
6]. As pancreatic fistulae are uncommon, most of the current literature and evidence are based on case series and case reports. Rana et al. in a study of 33 patients with low output external fistulae have shown spontaneous resolution in the fistulae in all patients over a mean of three months [
7]. However, endoscopic stent placement remains the primary therapeutic modality in high output fistulae. The aim of the present study was to determine outcomes of refractory pancreatic fistulae with endoscopic transpapillary stent placement and predictors of failure of therapy.
Go to :

MATERIALS AND METHODS
A retrospective review of prospectively collected data of patients with pancreatic fistulae with high output managed at the Department of Gastroenterology of a Tertiary Care Referral Center in Western India between January 2017 and December 2020 was carried out after obtaining approval from the Institutional Ethics Committee (no. EC 21/122). Consent waiver was received from the Ethics Committee. Patients with pancreatic ascites needing repeated paracentesis, pancreaticopleural fistula with need for intercostal drain placement and persistent drain output, or pancreaticocutaneous fistula with high output (> 200 mL/day) not responding to standard medical therapy and referred for endoscopic retrograde cholangiopancreatography (ERCP) were included in the analysis. A minimum 7 days of conservative management of fistula was required prior to consideration for intervention. Background history of acute pancreatitis or recurrent acute pancreatitis, trauma, presence of necrosis and evidence of chronic pancreatitis were noted. Details of clinical presentation, type of fistula, pre-endoscopic imaging with computed tomography (CT) scan of abdomen or magnetic resonance cholangiopancreatography (MRCP) (
Fig. 1), details of ERCP procedure including pancreatogram, type of stent placed, anatomy of pancreatic duct, site of leak, strictures in the pancreatic duct, bridging of leak using stent and periprocedural complications were also noted. Details of pre-procedure investigations including complete blood count, liver function tests and kidney function tests were obtained. Leak with ductal disruption seen on CT or MRCP was used as a guide for endoscopic intervention. Technical success was defined by successful main pancreatic duct cannulation and stent placement. Functional or clinical success was defined by clinical resolution of fistula after procedure with nil external drain, resolution of ascites, or pleural effusion. Details of additional procedures such as endoscopic ultrasound (EUS)-guided cystogastrostomy were also noted. Factors predicting failure of endoscopic therapy were analyzed.
 | Fig. 1Magnetic resonance cholangiopancreatography showing pancreatic leak (arrow) from the tail in a patient with ductal disruption and associated pseudocyst in the neck and proximal body of pancreas. CBD, common bile duct; PD, pancreatic duct; GB, gall bladder. 
|
Standard management protocol for patients presenting with pancreatic fistula includes management of underlying etiology such as acute pancreatitis with intravenous fluids, intravenous octreotide, nutritional supplementation and further plan based on imaging investigations with CT scan and MRCP as deemed necessary by treating physicians. Further management was decided by a multidisciplinary team based on clinical and imaging findings of ductal disruption. All ERCP procedures were done by endoscopists with experience of at least 500 procedures and skilled in both ERCP and EUS under monitored anaesthesia care or general anaesthesia as deemed appropriate. ERCP was done using standard duodenoscope (TJF Q180V; Olympus Corp., Tokyo, Japan) for all patients. Guide-wire assisted cannulation of the pancreatic duct was done using a Sphincterotome (CleverCut 3V; Olympus Corp.) and a VisiGlide 0.025” guidewire (Olympus Corp.). Pancreatogram was taken after injection of contrast to assess the site of disruption (
Fig. 2–
4). Pancreatic sphincterotomy was done and 5- or 7-Fr pancreatic stents (Cook Medical LLC, Bloomington, IN, USA) were placed in the pancreatic duct. For patients with pancreatic strictures, dilatation of stricture was done with Soehendra biliary dilators (5- and 7-Fr size; Cook Medical LLC). Wire guided cystotome (6 Fr; Endo-Flex [Medi-Globe], Rohrdorf, Germany) was used in patients with recalcitrant strictures. Attempt to bridge the leak was made for all patients. For patients with concomitant biliary obstruction, biliary stent placement was done in the same setting. After primary stent placement, patients were monitored for clinical improvement. Persistence of leak was defined by persistent ascites, pleural effusion, or percutaneous drain. In case of persistence of leak, stent exchange was done. For patients with pancreatic strictures, dilatation and stent exchange were done. For patients with pancreatic stricture, scheduled stent exchange was done at 3 to 6 months. In the event of non-resolving fistula, patient was referred for surgery. For patients with clinical improvement, nutritional rehabilitation was advised and patients were asked to follow up at 2 to 4 weeks. A repeat pancreatogram was taken at an interval of 6 to 12 weeks after the initial stent placement as the physician’s discretion. For patients with acute pancreatitis, pancreatic stent removal was done after pancreatogram showed resolution of leak. For patients with disconnected pancreatic duct syndrome (DPDS), attempts were made to bridge the leak on pancreatogram. For patients with DPDS and peripancreatic fluid collection, cystogastrostomy was done where indicated. Long-term double pigtail plastic stent placement was done for patients with DPDS who underwent cystogastrostomy.
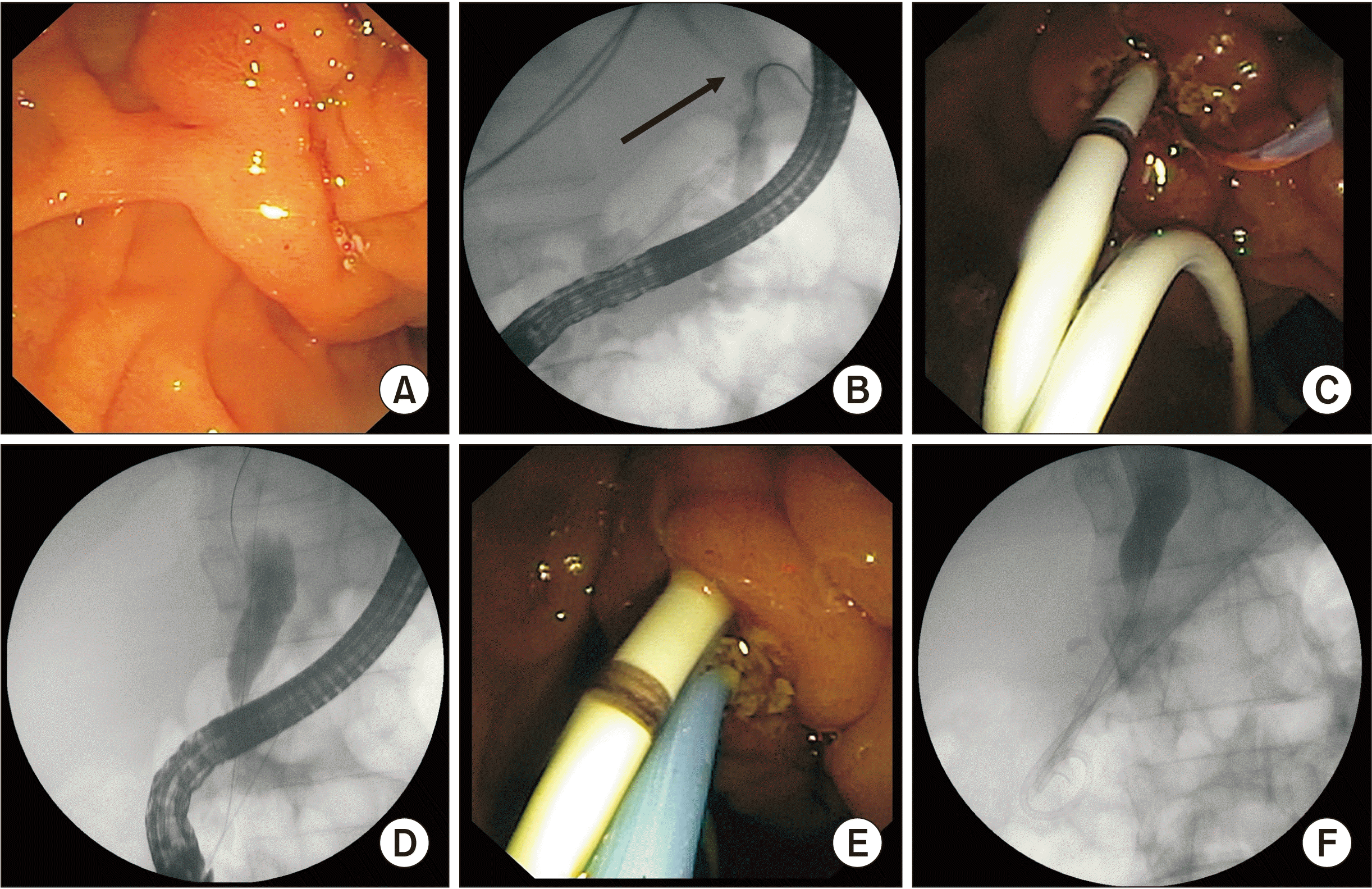 | Fig. 2(A) Native papilla in a patient with pancreatic pleural effusion and obstructive jaundice. (B) Pancreatogram showing leak in the tail of pancreas (arrow). (C) Pancreatic stent placement done to bridge the leak after sphincterotomy. (D) Stricture in distal common bile duct (CBD) on cholangiogram. (E) Plastic stent placement done in the distal CBD. (F) Fluoroscopic image showing stents in both CBD and pancreatic duct. 
|
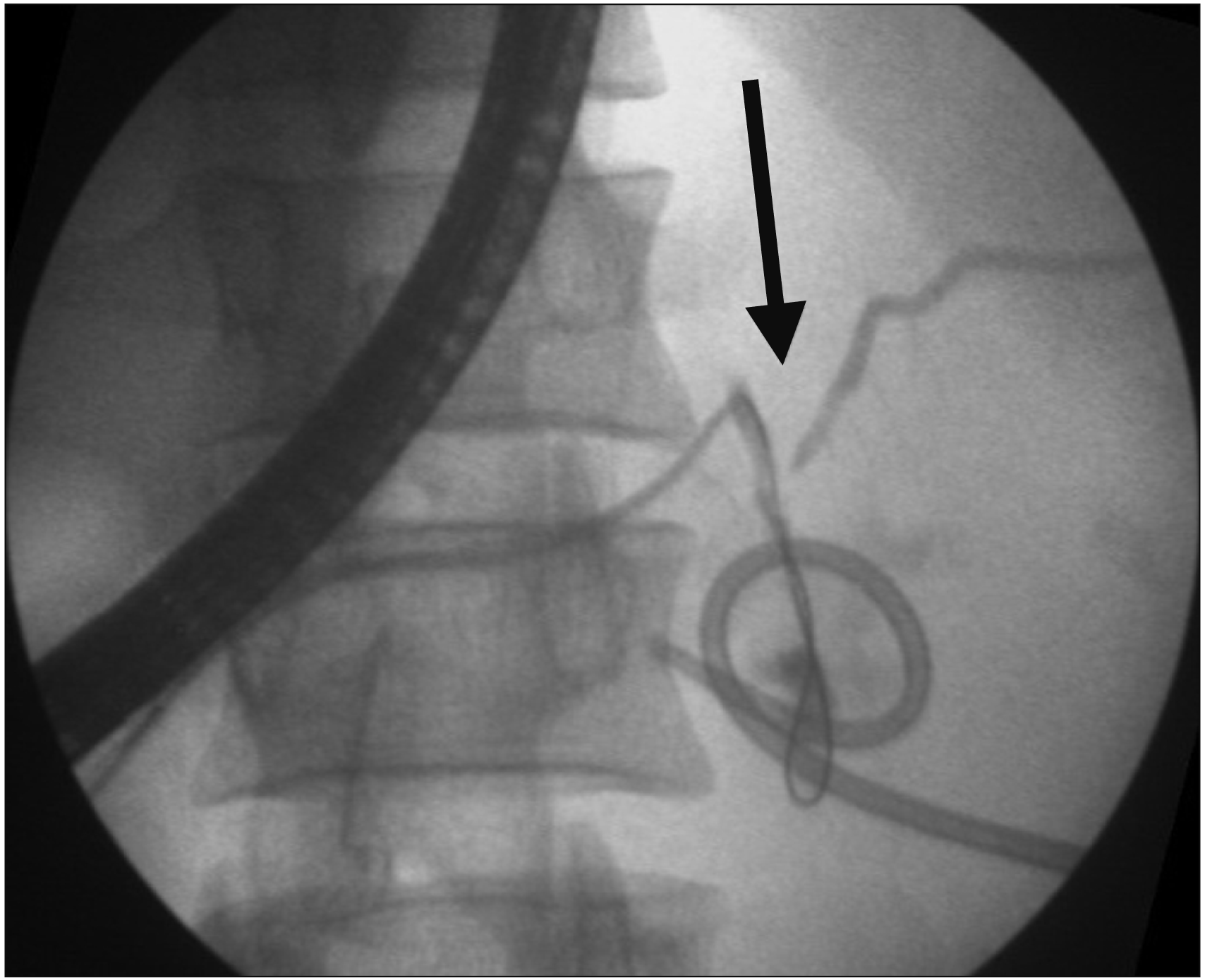 | Fig. 3Pancreatogram showing disruption in distal body of pancreas (arrow) having a percutaneous drain in peripancreatic collection, with wire passing into the collection. 
|
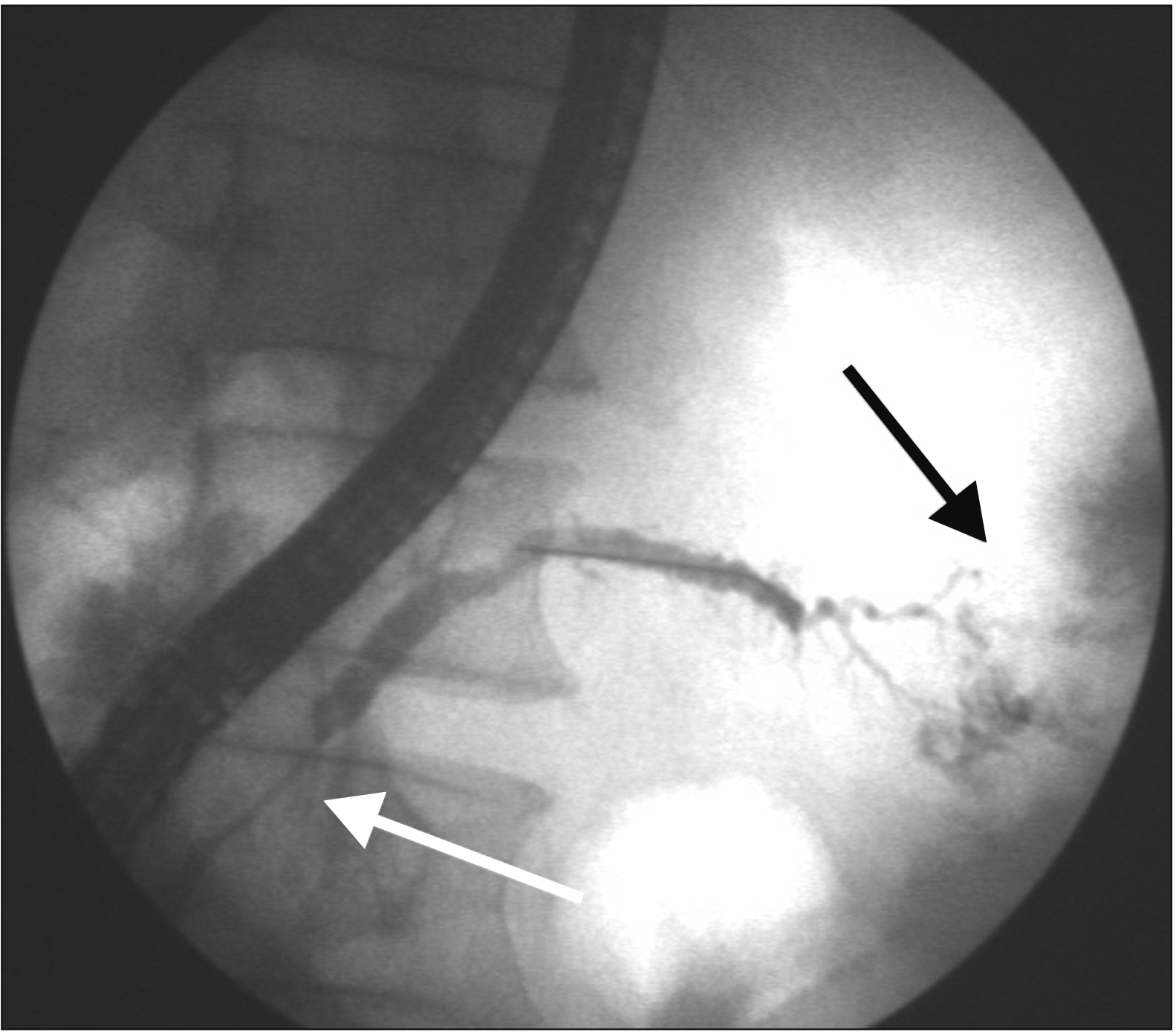 | Fig. 4Pancreatogram showing stricture in region of head (white arrow) with leak from tail of pancreas (black arrow). 
|
Statistical analysis
Categorical variables were analyzed using chi-squared test. Continuous variables were analyzed using Wilcoxon rank sum test. Univariate analysis and multivariate logistic regression were done to assess factors associated with non-resolution of pancreatic fistulae. Factors that might affect outcomes of stent placement were considered in multivariate analysis. A p-value of <0.05 was considered statistically significant. All statistical analyses were done using IBM SPSS version 23 (IBM Corp., Armonk, NY, USA).
Go to :

RESULTS
Sixty-eight patients with a mean age of 34 years and a median Charlson comorbidity index of 1 who presented with pancreatic fistulae not responding to standard medical therapy during the study period were included in the analysis. Demographic details are summarized in
Table 1. There were 62 (91.1%) males. High-output pancreatic fistulas were associated with chronic pancreatitis in 51.4% (35/68) of cases. Alcohol (52.9%; 36/68) was the most common cause of pancreatitis, followed by idiopathic pancreatitis (26.4%, 18/68). Seven patients had associated pancreas divisum. Internal fistulae and external fistulae were seen in 55 (80.9%) and 13 (19.1%) patients, respectively. It was found that 48.5% (33/68) and 30.9% (21/68) of patients had pancreatic ascites and pancreatic pleural effusion, respectively. One patient had pericardial effusion. In 45.5% (31/68) of cases, there was an associated pseudocyst, while 29.4% (20/68) of cases had associated walled-off pancreatic necrosis (WOPN). Postoperative fistulae were seen in seven patients (after pancreatic necrosectomy in 5 patients and post distal pancreatico-splenectomy for abdominal trauma in 2 patients). Percutaneous fistulae were seen in six patients, mostly secondary to percutaneous drainage of peripancreatic collections. While all patients underwent CT scan prior to intervention, 64.7% (44/68) had an MRCP prior to intervention. CT could identify the leak in 33 (48.5%) patients. MRCP could identify a leak in 88.6% (39/44) patients. In 8 (11.8%) patients, concomitant biliary obstruction was also seen. Median duration of symptoms prior to ERCP was 28 days (range, 14–62 days).
Table 1
|
Parameter |
Value |
|
Age (yr) |
|
|
Mean |
34.1 ± 11.1 |
|
Median (IQR) |
34 (3–72) |
|
Sex (male) |
62/68 (91.1) |
|
Charlson comorbidity index |
1 (0–7) |
|
Type of pancreatitis |
|
|
Chronic pancreatitis |
35/68 (51.4) |
|
Acute pancreatitis |
26/68 (38.2) |
|
Traumatic pancreatitis |
7/68 (10.3) |
|
Leak detected on CT scan |
16/68 (23.5) |
|
Leak detected on MRCP |
34/44 (77.3) |
|
Multiple sites of leak |
15/62 (24.2) |
|
Associated pancreatic duct stricture |
23/63 (36.5) |
|
Technical success |
63/68 (92.6) |
|
Functional success (clinical success at 6 weeks) |
52/68 (76.4) |

ERCP was technically successful in 92.6% (63/68) patients. Pancreatic sphincterotomy was done in all (100%) patients. Stenting from the minor papilla was successful in 85.7% (6/7) patients. Leak was visualised in 98.4% (62/63) patients (
Fig. 2–
4). Pancreatic body was the most common site of leak, seen in 69.8% (44/63) patients. Multiple sites of leak were seen in 23.8% (15/63) patients. Stricture with narrowing in the pancreatic duct was seen in 36.5% (23/63) patients. In 65.1% (41/63) patients, 7-Fr stent was placed. In 34.9% (22/63) patients, 5-Fr stent was placed. In 30.6% (19/62) patients, stent could not be placed beyond the leak. Reasons for inability to place stent beyond leak included leak in tail of pancreas or distal cut end of pancreas postoperatively (11/19), collapsed distal duct with inability to pass guide-wire (3/19), disconnected pancreatic duct (3/19) and stricture with inability to pass guide-wire (2/19). Complete ductal disruption with DPDS was seen in six patients. Of patients with DPDS, four underwent EUS-guided cystogastrostomy for peripancreatic fluid collections, while two had a percutaneous drain in situ. Double pigtail plastic stents were placed in the cyst cavity in all patients with DPDS who underwent cystogastrostomy. The location of DPDS was neck in one patient, body in three patients and tail in two patients. Leak was crossed in three patients. Stent was placed to bridge the leak. Resolution of seen in three patients with DPDS (leak was bridged in two, leak was not bridged in one). One patient was lost to follow-up. Functional success with resolution of leak was documented clinically in 86.6% (52/60) patients. Based on intention to treat analysis, 76.4% (52/68) patients had clinical success following pancreatic stent placement. Three patients were lost to follow-up. Two patients with DPDS did not show resolution of the fistula. Of these two patients with DPDS, one patient underwent surgery for persistent leak from body-tail junction of the pancreas. A total of seven (10.3%) patients underwent concomitant endoscopic cystogastrostomy successfully.
Outcome of pancreatic fistula at six weeks showed no significant association with age, sex, underlying chronic pancreatitis, site of leak, presence of pancreatic stricture, common bile duct (CBD) stricture, pancreatic ascites, pancreatic pleural effusion, presence of pseudocyst or WOPN, multiple sites of leak, serum albumin level, total bilirubin level, or serum creatinine level. Univariate and multivariate analyses revealed that stent placement beyond site of leak was the only factor associated with resolution at six weeks (OR: 6.5, 95% CI: 1.211–34.94;
p = 0.03) (
Table 2,
Fig. 5). Mild exacerbation of pancreatitis was the only complication, seen in 8.1% (5/62) patients. One patient had stent migration which was passed out spontaneously. Three (4.4%) patients included in this study died (2 without resolution of fistula secondary to abdominal sepsis, 1 after fistula resolution due to pneumonia and urinary tract infection with septic shock). Of six patients without resolution of fistula, four underwent surgery with distal pancreatectomy. Two other patients were managed conservatively and resolved over three months. Stent removal with pancreatogram was done in 85.0% (51/60) patients on follow-up. Persistent small leak occurred in six patients. However, there was no clinical evidence of fistula.
 | Fig. 5Flow diagram of study outcomes. PD, pancreatic duct; ERCP, endoscopic retrograde cholangiopancreatography. 
|
Table 2
Results of univariate and multivariate analyses
|
Parameter |
Resolved |
Not resolved |
p-value (univariate) |
p-value (multivariate) |
|
Sex |
|
|
0.28 |
|
|
Male |
48/56 |
8/56 |
|
|
|
Female |
4/4 |
0/4 |
|
|
|
Age (yr) |
34.08 ± 11.35 |
40.63 ± 15.06 |
0.27 |
|
|
Background CP |
|
|
0.84 |
0.86 (OR: 1.17, 95% CI: 0.207–6.622) |
|
Yes |
28/32 |
4/32 |
|
|
|
No |
24/28 |
4/28 |
|
|
|
Primary site of fistula |
|
|
0.35 |
0.31 (OR: 1.82, 95% CI: 0.34–5.41) |
|
Internal |
42/47 |
5/47 |
|
|
|
External |
10/13 |
3/13 |
|
|
|
Presence of pseudocyst |
|
|
0.35 |
|
|
Yes |
27/29 |
2/29 |
|
|
|
No |
25/31 |
6/31 |
|
|
|
Walled off necrosis |
|
|
0.74 |
|
|
Yes |
16/18 |
2/18 |
|
|
|
No |
36/42 |
6/42 |
|
|
|
Fistula number |
|
|
0.67 |
0.42 (OR: 2.81, 95% CI: 0.231–34.249) |
|
Single |
38/45 |
7/45 |
|
|
|
Multiple |
14/15 |
1/15 |
|
|
|
Pancreatic duct stricture |
|
|
0.13 |
0.11 (OR: 0.141, 95% CI: 0.013–1.512) |
|
Yes |
21/22 |
1/22 |
|
|
|
No |
31/38 |
7/38 |
|
|
|
CBD stricture |
|
|
0.31 |
0.99 |
|
Yes |
6/6 |
0/6 |
|
|
|
No |
46/54 |
8/54 |
|
|
|
Size of stent |
|
|
0.87 |
0.40 (OR: 0.463, 95% CI: 0.076–2.808) |
|
5 Fr |
18/21 |
3/21 |
|
|
|
7 Fr |
34/39 |
5/39 |
|
|
|
DPDS |
|
|
0.06 |
|
|
Yes |
3/5 |
2/5 |
|
|
|
No |
49/55 |
6/55 |
|
|
|
Stent placed beyond site of leak |
|
|
0.04 |
0.03* (OR: 6.5, 95% CI: 1.211–34.94) |
|
Yes |
38/41 |
3/41 |
|
|
|
No |
14/19 |
5/19 |
|
|
|
Albumin levels (g%) |
3.01 ± 0.59 |
2.66 ± 0.54 |
0.12 |
|
|
Creatinine levels (mg%) |
1.12 ± 0.34 |
1.16 ± 0.22 |
0.70 |
|

Go to :

DISCUSSION
Pancreatic fistulae, albeit uncommon, remain a perplexing clinical problem. Our study demonstrated technical success of endoscopic management in high output IPF and EPF in 92.6% patients. Resolution of fistulae at 6 weeks occurred in 86.6% patients with technically successful pancreatic stent placement. Overall clinical success was 76.4%. Endoscopic transluminal drainage of peripancreatic collections was done in 10% patients. Surgery was needed in a small proportion for treatment of fistulae. Stent placement beyond site of leak was the only factor associated with resolution of fistula.
All patients underwent CECT in our study. However, leak was demonstrable on CT in only 48.5% patients. A previous study by O’toole et al. [
8] has demonstrated 50% detection of leak on helical CT in patients with pancreatic fistula. This yield increased further to 94% when combined with MRCP. MRCP is useful for patients for diagnosis of leak with high sensitivity (~90%) and specificity. However, real time assessment of pancreatic secretion was not possible with MRCP. Secretin stimulated MRCP may aid dynamic characterization of pancreatic leak. Unlike ERCP, secretin stimulated MRCP can be used to evaluate duct upstream from site of complete disruption [
9]. However, sensitivity of secretin stimulated MRCP is lesser than that of ERCP. In our series, MRCP was able to detect the fistula in 88.6% patients.
Therapy in pancreatic fistulas, especially those secondary to acute pancreatitis, is supportive. This includes nutritional support, intravenous hydration, skin care in cases of external fistulae and use of somatostatin analogues [
10]. Spontaneous resolution is known to occur in 50%–60% IPF and 80% EPF. Persistence of pancreatic fistulous output has implications on fluid management, nutritional optimization, risk of infectious complications, complex wound care and overall poor quality of life. Somatostatin analogues in a previous meta-analysis of 297 patients with gastrointestinal fistulae showed no significant benefit on rates of pancreatic fistula closure (OR: 1.52, 95% CI: 0.88–2.61) [
11]. However, some trials have demonstrated a significant reduction in output from fistula without any increased rate of fistula closure in patients receiving somatostatin or its analogues [
12]. A previous meta-analysis of 1,700 patients showed that octreotide given in the postoperative period reduced risk of postoperative pancreatic fistulae and hospital stay [
13]. However, another recent meta-analysis in 2019 including 2,000 patients showed no benefit of prophylactic use of somatostatin analogues in reducing rates of postoperative pancreatic fistulae [
14]. In our series, we only included patients who did not respond to initial therapy with persistent drain output or refilling of ascites or pleural effusion.
Endoscopic transpapillary pancreatic stent placement is associated with creation of a low resistance pathway for drainage of pancreatic secretions rather than through the disrupted duct, which might be one of the factors associated with possible resolution [
15]. Other possible sites of resistance like strictures and calculi can also be bypassed by stent placement, leading to decrease in the ductal pressure and possible resolution of pancreatic leak. In our study, all patients with successful wire-guided pancreatic duct cannulation underwent pancreatic sphincterotomy. In addition, 36.5% patients had presence of a pancreatic stricture on pancreatogram. Presence of pancreatic stricture did not impact the outcome of fistula resolution. Concomitant biliary obstruction is known to occur in 3% to 46% patients with chronic pancreatitis [
16]. In patients with acute pancreatitis, biliary obstruction in the form of calculi or sludge or strictures may be seen. Outcomes of pancreatic fistula at 6 weeks showed no correlation with biliary obstruction or need of biliary stenting.
In a previous study by Das et al. [
17] enrolling 107 patients with pancreatic duct disruption, technical success of the ERCP was achieved in 96% patients. The leak was bridged by stent placement in 44% patients. Functional success was seen in 75% patients. Etiology for pancreatic fistula was acute pancreatitis in 36% patients, postoperative in 31% and chronic pancreatitis in 29%. Non-acute pancreatitis etiologies and absence of complete duct disruption were predictors of therapeutic success in these patients. Only 33% patients had received somatostatin analogues prior to ERCP. Also, the majority (89%) of the cases were peripancreatic fluid collections. The fistula output was not documented in that study. In our series, Chronic pancreatitis was the primary etiology in 51.4% patients. Only bridging the leak by pancreatic stent placement was predictive of resolution of fistulae. The majority of our patients had pancreatic ascites (50%) or pancreatic pleural effusion (45.5%). Almost all patients had received conservative management with somatostatin analogues. Only patients with persistent high output fistulae (> 200 mL) or ascites needing repeated paracentesis were included in the analysis.
In a previous study from India of 53 patients with pancreatic ascites and pleural effusions, leak was demonstrable in 37.7% patients [
18]. Site of leak and stent placement beyond leak were independently associated with clinical success. In addition, 73.6% patients showed complete resolution of ascites and pleural effusion. However, that study did not mention the severity of fistula. Also, while clinical success was similar to our study, leak was seen on ERCP in 98.6% patients of our study. In another previous study by Telford et al. [
19] enrolling 43 patients with pancreatic ductal disruption, bridging the site of leak with stent was the only factor that showed correlation with successful clinical outcome on multivariate analysis. Varadarajulu et al. [
20] demonstrated that in addition to bridging the site of leak, partial duct disruption was associated with favorable outcomes on multivariate analysis after endoscopic pancreatic stent placement. A previous review evaluating outcomes of closure of pancreatic fistula by transpapillary stent placement showed functional success in 71% cases [
21]. In another study, Tilara et al. [
22] have shown that EUS-guided transmural drainage (EUS-TD) is a potential alternative in postoperative pancreatic fistulae. In a previous study of 196 patients with postoperative pancreatic fistulae (132 with peripancreatic collections and 64 without), there was a trend towards a higher rate of clinical resolution (85%) as compared to surgery (41%) and percutaneous intervention (64%) [
23]. In another recent study, technical and clinical success rates of EUS-TD were 100% and 97%, respectively, with 47% patients undergoing EUS-TD early after surgery (< 15 days) [
24]. In our series, seven patients underwent concomitant cystogastrostomy.
The rate of complications in the present study was similar to rates reported previously [
15,
18]. Exacerbation of pain of pancreatitis was reported in 8%. Migration although is known to occur in 1.5% of patients undergoing pancreatic stent placement [
25]. Only one patient in our series had distal migration of stent. Infectious complications may occur, leading to severe sepsis. Two patients in our series developed infection of peri-pancreatic collections and succumbed. One patient with resolution of fistula developed lower respiratory tract infection and urinary tract infection leading to sepsis and septic shock with eventual mortality.
The strength of our study was that we included patients with persistent high-output fistulae who failed supportive therapy. In addition, both clinical and endoscopic outcomes were documented, with repeat pancreatogram being available for most patients. Limitations of this study include its small sample size and a retrospective study design which could restrict the study’s generalizability. However, our study results agree with previous studies on pancreatic fistulae.
In conclusion, high-output pancreatic fistulae can be treated using endoscopic modalities, showing technical success and overall clinical success in 92.6% and 76.4% cases, respectively. Bridging the site of leak by endoscopic transpapillary stent placement is an effective and safe approach for the management of IPF and EPF not responding to a supportive therapy.
Go to :







 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download