INTRODUCTION
Postoperative fluid collection (POFC) is a common complication of a pancreaticoduodenectomy (PD) with high morbidity rates. Although mortality rates associated with pancreatic resection have decreased over recent decades to less than 1% in high-volume centers [
1], there has been no significant decrease in the level of morbidity among these cases [
2,
3]. Various studies have shown from routine computerized tomography (CT) scans that 9%–69% of PD patients develop POFC [
4,
5], among which 8%–22% of cases require radiologic drainage [
6,
7]. Indications for drainage include infected or enlarging POFC and symptoms such as abdominal pain, gastric outlet obstruction, biliary obstruction, and fistulation [
8]. Surgical drainage and percutaneous drainage have traditionally been the mainstay of POFC management. However, with recent technical advances and the introduction of linear endoscopic ultrasound (EUS) in the 1990s, EUS-guided drainage has emerged as an alternative to traditional drainage methods [
9]. EUS-guided drainage was first described in 1992 [
10]. The use of ultrasound in this methodology enables the identification and avoidance of vessels, measurement of the distance from the gastric lumen to the site of fluid collection, and localization of non-bulging fluid collections, leading to higher success rates and lower complication rates compared to conventional transmural drainage [
11,
12].
As the clinical experience with EUS-guided drainage procedure has accumulated, many studies have reported its outcomes in comparison with the percutaneous approach, especially in pancreatic pseudocysts. However, to the best of our knowledge, no prior study has thoroughly analyzed clinical outcomes of these two drainage procedures for treating POFC complications in PD patients. Thus, the objective of this study was to compare outcomes of EUS-guided drainage and percutaneous catheter drainage (PCD) in patients who experienced this adverse event after PD.
Go to :

MATERIALS AND METHODS
Electronic medical records of 2,160 patients who had undergone a PD surgery in our hospital between January 2015 and June 2019 at our center were retrospectively reviewed. Among these cases, 155 had a confirmed POFC by CT scanning. They were further screened for enrolment in our study cohort. Ninety-seven of these patients were treated by conservative management only, such as nil per os (NPO), total parenteral nutrition, and intravenous (IV) antibiotics. Twenty-one of these 155 cases underwent PCD whereas 32 received EUS-guided drainage as the primary drainage procedure. One patient underwent both drainage procedures simultaneously due to separately loculated fluid collections. This case was excluded from further analysis. There were two cases of embolization due to active bleeding from pseudoaneurysm. There were two cases of reoperation (abscess drainage and irrigation) for two patients with unstable vital signs. These four cases received embolization or reoperation without prior or concomitant intervention. Finally, 32 patients who received EUS-guided drainage and 21 patients who underwent PCD were selected as our study population (
Fig. 1). All follow-up electronic medical records up to December 2019 were reviewed for these cases. Demographic data, symptoms, lab data, fluid culture results, antibiotic regimens, POFC diameters on CT image, and drainage outcomes were analyzed for these included patients. In terms of the PD procedure, a duct to mucosa method with an internal stent was routinely performed for pancreaticojejunostomies. Two to three surgical drains were routinely placed around pancreaticojejunostomy, choledochojejunostomy, and gastrojejunostomy anastomosis depending on the surgeon’s preference and judgment. Daily drain amylase and lipase levels were checked until postoperative day 3. If there was no biochemical leak or clinical derangement from the routine postoperative course and the drain color was serous, drains were removed starting from postoperative day 3. If there were any abnormalities in drain amylase/lipase levels, drain color/amount, and clinical signs or symptoms, drains were kept in place for as long as they were needed.
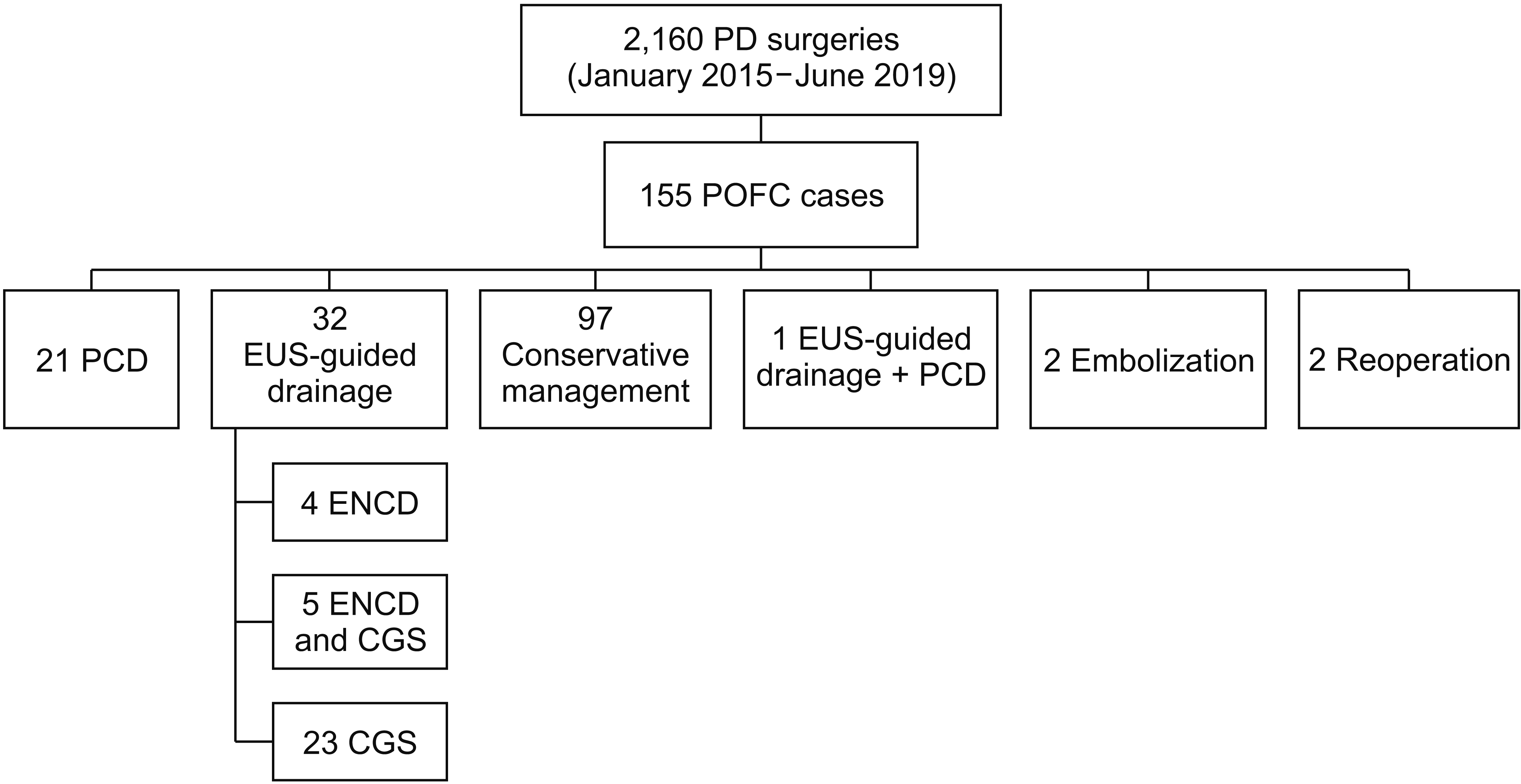 | Fig. 1
Selection of study population. Of 2,160 patients who underwent pancreaticoduodenectomy (PD) between January 2015 and June 2019, there were 155 cases of postoperative fluid collection (POFC). Among them, 32 cases of percutaneous catheter drainage (PCD)
and 21 cases of endoscopic ultrasound (EUS)-guided drainage were selected as our study population. ENCD, endoscopic nasocystic drainage; CGS, cystogastrostomy.

|
A routine CT check was done on postoperative day 5 which helped surgeons determine the optimal time for drain removal or the need for additional management and intervention. Indications for drainage included evidence for fluid collection on CT, inflammatory marker elevation, or symptoms such as fever, abdominal pain, and nausea/vomiting. The timing and type of drainage was determined by consultation between the surgeon, gastroenterologist, and interventional radiologist. Given the specificity of pancreas resection, there were several considerations to make in the timing and type of drainage, such as drain amylase and lipase levels, POFC diameter, severity of symptoms, inflammatory marker levels, and vital signs and CT findings. POFC findings on CT scans did not always mean that drainage was needed. Infection sign including high fever/pain/leukocytosis is the most important criterion in judging a need for a drainage procedure. In addition, whether it could be controlled with IV antibiotics only, whether the procedure was possible considering location of POFC, and whether the size was increasing were also factors that were taken into account.
This study was approved by our center’s Institutional Review Board (IRB number: S2020-0679-0001).
EUS-guided drainage
Prior to conducting the EUS-guided drainage procedure, all patients were administered IV midazolam for sedation and meperidine for pain control. EUS was performed using a linear array echoendoscope with fluoroscopic guidance. Once the site of the POFC was determined, the stomach wall was punctured using a needle. The contrast media was then injected to confirm this location. The endoscopist next inserted a guidewire, removed the needle, and dilated the fistula tract. Either a nasocystic drainage catheter, a fully covered self-expanding metal stent, or a double pigtail plastic stent was then inserted through the dilated tract. An endoscopic nasocystic drainage (ENCD) was sometimes additionally inserted at the endoscopist’s discretion during EUS-guided stent insertion. The most important purpose of ENCD was to maintain patency of the internal stent. Additionally, POFCs were often thick. Placing an ENCD could ensure more effective drainage. It could also allow POFC cultures to be taken for the purpose of determining appropriate antibiotics.
Following EUS-guided cystogastrostomy (EUS-CGS), the patient underwent a follow-up CT at about four weeks after the procedure. The stent was removed once resolution of the POFC was confirmed.
Percutaneous drainage
During PCD interventions, patients were given IV meperidine prior to the procedure for pain control. A needle was inserted into the fluid collection under ultrasonographic guidance. Contrast media was then injected to confirm the POFC location. A pigtail catheter was placed after dilation of the percutaneous tract. A bile bag was connected to the pigtail catheter and daily output was recorded.
Follow-up procedures and parameters
Outcomes were measured using several parameters, including technical success, clinical success, reintervention rates, adverse events, post-procedural IV antibiotic duration, and post-procedural hospital stay. Technical success was defined as the successful placement of a drain stent or catheter at the intended POFC site. Clinical success was analyzed based on POFC diameter decrease of more than 50% on follow-up CT image within eight weeks. The number and interval of CT scan follow-ups and time of discharge were based on the surgeon’s judgement. Reintervention was defined as the need for a subsequent drainage or other types of interventions due to persistent symptoms associated with a residual POFC. Adverse events were unwanted events causing harm to the patient that occurred because of the drainage procedure. They included hemorrhage, perforation, stent migration, and sepsis due to the procedure.
Statistical analysis
All statistical analyses were conducted using SPSS version 18.0 (IBM Corp., Armonk, NY, USA). Univariate analysis was performed using a chi-squared test for categorical variables. A t-test was performed for continuous variables with an even distribution and a Mann-Whitney test was performed for continuous variables with an uneven distribution. A p-value of less than 0.05 was considered significant. Data values are presented as mean ± standard deviation.
Go to :

DISCUSSION
POFC is a major cause of postoperative morbidity and mortality following PD surgery. In our center, 7.1% of PD patients developed a POFC complication, which was visible on a postoperative CT scan. This POFC frequency was lower than the range of 9%–19% reported in previous studies [
13-
15]. Drainage procedures were required in 2.5% of PD cases in our hospital, which was again lower than previously reported rates of 8%–22% [
6,
7]. One possible explanation for these discrepancies is that our institution is a high volume, specialized center with an annual average of 450 PD cases.
Percutaneous or surgical interventions have been the traditional management of symptomatic POFCs. Since the 1990s, however, EUS-guided drainage has emerged as a new option for these interventions. It has been reported to be effective and safe for peripancreatic fluid drainage in multiple studies with clinical success rates of 70%–87% and complication rates of 11%–34% [
11,
16-
18]. Several studies have compared these two drainage approaches for POFC after a distal pancreatectomy [
19-
23]. But to the best of our knowledge, our current investigation is the first to compare in detail PCD and EUS-guided drainage procedures for POFC complications arising after PD. We believe our findings provide valuable information for optimal drainage modality after PD.
There are several points to consider for drainage in POFC after PD. High drain amylase and lipase levels, larger POFC diameter, unimproved symptoms, high inflammatory markers, and remarkable infection signs necessitate drainage management. Based on CT findings, we could expect whether routine surgical drains would be effective in managing POFC if it was present. If they were deemed to be ineffective, the next step would be deciding the type of drainage. If the patient had unstable vitals such as low blood pressure, high fever, and tachycardia with a concomitant postoperative abscess, surgical drainage should be considered. If the patient was stable enough, percutaneous or endoscopic drainage was considered. The location of the fluid collection was the most important factor when deciding between the two methods. If the POFC was right beneath the peritoneum, then the percutaneous method was preferred. If the POFC was adjacent to the stomach and difficult to approach percutaneously without injuring the bowel or spleen, then EUS-CGS was preferred. We compared the two study groups’ clinical characteristics and outcomes in this study.
The average POFC diameter was greater in the PCD group in our study (11.4 cm in PCD vs. 8.2 cm in EUS). In other studies, POFCs treated using EUS-guided drainage have varied sizes ranging from 7.9 cm to 9.6 cm [
22,
24]. Kwon et al. [
19] have described a mean cyst size of 10 cm for patients undergoing PCD and 8.9 cm in those treated using EUS-guided drainage after a partial pancreatectomy. EUS guided drainage is feasible only if fluid collection is adjacent to the stomach while percutaneous drainage is easier if the collection is located superficially, near the abdominal wall. Most POFCs that underwent EUS drainage in our present patient population were loculated in the lesser sac near the pancreas head resection site. On the other hand, many POFCs that underwent PCD drainage had variable locations, including perihepatic and lower abdomen sites, leading to differences in diameter in our study.
We use prophylactic IV cefotaxime and metronidazole for PD at our center. However, piperacillin/tazobactam, meropenem, metronidazole, vancomycin, and imipenem/cilastatin are most frequently used antibiotics after drainage procedures. Cefotaxime and metronidazole cannot cover Enterococcus or Pseudomonas, which were commonly detected in our current fluid samples. Thus, broader antibiotic coverages were needed. The mean duration of IV antibiotic administration from the day of intervention in our present series was 8.1 ± 4.3 days for the EUS group and 12.4 ± 7.4 days for the PCD group (p = 0.01). The shorter antibiotics usage duration in the EUS group might be due to faster resolution of symptoms and shorter hospital stay in this group because it was difficult to perform IV administration after discharge.
The technical success rate was 96.9% for the EUS group and 100% for the PCD group. There was only one case of technical failure in the EUS group due to remnant gastric content. In our present study cohort, 32 patients had undergone EUS-guided drainage for a POFC. Four of these cases received an ENCD only because no wall maturation was observed in three patients and a fistula was observed in the remaining patient. Our success rates were similar to those described by other studies. Technical success rates of 93.6% to 100% for EUS-guided drainage 97.4% to 100% for PCD have been described [
19,
20,
22,
25,
26]. Thus, both drainage procedures can be done with a high accuracy using current techniques with adequate clinical experience.
We analyzed clinical success in our current study as greater than 50% decrease in the POFC diameter on a CT scan within eight weeks of the drainage procedure. The definition of clinical success in relation to POFC drainage varied across the published literature. Gupta et al. [
24] and Varadarajulu et al. [
22] defined this as the resolution of fluid collection on abdominal imaging, in association with clinical resolution of symptoms at eight weeks of follow-up. Their definition was similar to ours. Gupta et al. [
24] and Varadarajulu et al. [
22] reported clinical success rates of 79% and 100% for EUS-guided drainage, respectively. The present study had a clinical success rate of 87.5%.
There were two (6.3%) cases of adverse events in our current EUS-guided drainage group but none in the PCD group. Other reports have also indicated low complication rates from POFC drainage methods. Song et al. [
20] and Kwon et al. [
19] have reported zero adverse events from EUS-guided drainage after distal pancreatectomy. One of the reasons is that EUS-guidance has an easier anatomical approach for POFC drain intervention. However, other authors have described higher rates, with 14% being the highest reported [
19,
20,
22,
24-
26]. Our center’s adverse event rates after EUS-guided drainage are within reasonable range compared to these previous studies.
We observed 4 (12.5%) and 7 (33.3%) reintervention cases in our EUS-guided drainage group and our PCD patients, respectively. Previous studies have reported reintervention rates ranging from 14.5% [
20] to 38% [
25] with EUS-guided drainage and 19.2% [
23] to 41% [
26] with PCD involving salvage drainage procedures [
19,
22,
24].
In our current study population, the post-procedural hospital stay was significantly shorter for the EUS group. Because EUS-CGS is an internal drain, surgeons can safely discharge patients with the stent in situ after clinical symptoms have improved and the POFC has decreased in size. On the other hand, PCD involves an external drain that needs to be removed prior to hospital discharge which can be complicated by the risk of residual fluid collection, often leading to a longer hospital stay. Clinicians are also typically more conservative in relation to discharging PCD patients. They often wait until drainage is at least near-complete or there has been complete resolution of the POFC before catheter removal. Notably, however, in a match-controlled cohort study of patients who received pancreatic resection, Al Efishat et al. [
26] found no significant difference in hospital stay between the two drainage methods (4 days with EUS-guided drainage vs. 3 days with PCD,
p = 0.76). Azeem et al. [
23] have analyzed 48 patients with POFC after a distal pancreatectomy and observed a longer median post-procedural hospital stay after primary PCD (5.5 days for PCD vs. 2 days for EUS-guided drainage,
p = 0.05). However, both groups in their study showed significantly shorter hospital stays than our present study results. The timing of a hospital discharge can be affected by many factors such as medical cost, insurance coverage, and medical accessibility, which can explain these different results. In the study by Azeem et al. [
23], the PCD catheter was in place for a mean of 20 days. It was for a mean of 33 days in Efishat’s study [
26]. Taking into consideration the short post procedural hospital stay, we could deduce that patients were discharged shortly after drainage procedure and that their drains were then probably removed during a subsequent out-patient follow-up. On the other hand, many PCD patients at our hospital tend to be discharged after PCD removal because of negative effects of leaving the drain in place on patient’s quality of life (QOL) and the risk of an ascending infection.
This study has some limitations. First, there were inherent limitations of its retrospective and non-randomized design. Another limitation was that we only analyzed patients from our single, academic, tertiary hospital who had been treated by experienced endoscopists and hepatobiliary surgeons. Therefore, our results might not be applicable to smaller centers. Finally, the decision on whether to conduct a PCD or an EUS-guided drainage largely depended on the location of the POFC. In many of our included cases, peripancreatic fluid collections near the stomach were treated using EUS-guided drainage while POFCs with relatively superficial locations underwent PCD. Certain fluid collection locations might be related to better outcomes. However, this was not fully taken into consideration in our present analyses. More analysis regarding the difference in POFC diameter and location is necessary to evidentiary support the shorter length of stay of EUS guided drainage group. However, EUS-guided drainage procedure for fluid collection after PD is still in its early stages. As a result, there was a relatively small study population. Moreover, considering the retrospective nature and non-randomized grouping of patients which are major limitations of this study, it seems difficult to perform an additional analysis for this issue. Nevertheless, we find this study worthwhile in that this is the first study to perform the analysis for initial experiences for EUS guided drainage after PD to the best of our knowledge. As drainage methods are mostly determined by the location of the fluid collection, we believe the longer hospital stay of the PCD group is mainly due to the removal timing of the PCD, not because of the larger size of fluid collection. In this study, we compared two modalities of drainage after PD for the first time to our knowledge. Literature is still scarce on this subject. A multicenter, randomized, prospective study comparing the EUS-guided drainage and PCD approaches is warranted in the future to validate our findings and optimize treatments for POFC complications arising from a PD.
In conclusion, technical and clinical success, reintervention rates, and adverse event rates are comparable between EUS-guided drainage and PCD catheter drainage for the treatment of a POFC after PD. However, the EUS-guided approach is associated with a shorter post procedural IV antibiotic usage and hospital stay.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download