Abstract
Backgrounds/Aims
Bile duct stones (BDS) can be managed either prior to laparoscopic cholecystectomy (LC) using endoscopic retrograde cholangiopancreatography (ERCP) or with laparoscopic bile duct exploration (LBDE) at the time of LC. The latter is underutilised. The aim of this study was to use the dataset of the previously performed CholeS study to investigate LBDE hospital volumes, LBDE-to-LC rates, and LBDE outcomes.
Methods
Data from 166 United Kingdom/Republic of Ireland hospitals were used to study the utilisation of LBDE in LC patients.
Results
Of 8,820 LCs performed, 932 patients (10.6%) underwent preoperative ERCP and 256 patients (2.9%) underwent LBDE. Of the 256 patients who underwent LBDE, 73 patients (28.5%) had undergone prior ERCP and 112 patients (43.8%) had undergone prior magnetic resonance cholangiopancreatography. Fifteen (9.0%) of the 166 included hospitals performed less than five LBDEs in the two-month study period. LBDEs were mainly performed by upper gastrointestinal surgeons (84.4%) and colorectal surgeons (10.0%). Eighty-seven percent of the LBDEs were performed by consultants and 13.0% were performed by trainees. The laparoscopic-to-open conversion rate was 12.5%. The median operation time was 111 minutes (range: 75–155 minutes). Median hospital stay was 6 days (range: 4–11 days) for emergency LBDEs and 1 day (range: 1–4 days) for elective LBDEs. Overall morbidity was 21.5%. Bile leak rate was 5.3%. Thirty-day readmission and mortality rates were 12.1% and 0.4%, respectively.
Bile duct stones (BDS) are present in 10% to 20% of patients with symptomatic gallstones [1]. They require treatment to reduce the risk of further morbidity [1]. There are two accepted treatment methods: a two-stage approach and a single-stage approach. In the more commonly performed two-stage approach, preoperative magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP) are carried out prior to laparoscopic cholecystectomy (LC). In the single-stage approach, which is less common, upfront LC is performed along with laparoscopic bile duct exploration (LBDE). A recent meta-analysis by Lyu et al. [2], using 12 randomised controlled studies (n = 1,545), suggested patients who underwent ERCP + LC had higher stone clearance rates and a lower rate of bile leakage than those who underwent LC + LBDE . However, the latter had lower rates of postoperative pancreatitis and reduced length of stay [2]. Overall morbidity and mortality rates were similar [2].
Although the single-stage approach is supported by recent evidence, it is not commonly used across the United Kingdom (UK) or the Republic of Ireland (RoI). Reasons for this remain unclear. The lack of data on LDBE outcomes from large multicentre studies might be a contributing factor. Most published series are from single institutions or low-volume centres with outcomes that are difficult to generalise. The aim of the present study was to analyse the CholeS study dataset to examine LBDE hospital volumes, LBDE-to-LC rates and LBDE outcomes.
The CholeS study was a multicentre, prospective population-based cohort study that investigated variations in practice and outcomes of cholecystectomy [3-5]. It did not require registration since anonymous, observational data were collected. Research ethics approval was not required; this was confirmed using the online National Research Ethics Service (NRES) decision tool (http://www.hra-decisiontools.org.uk/research/). It was not necessary to obtain consent from the included patients since no identifiable information was collected and the study did not affect clinical care. The study was registered as a clinical audit or service evaluation at each participating hospital under the supervision of a named senior investigator (a consultant surgeon). The study adhered to the ethical standards laid down in the Declaration of Helsinki of 1975 (revised 2013). Data were collected for 8,820 LCs and 256 LBDEs from 166 hospitals across the UK and RoI in March and April of 2014. The data were reviewed by independent data validation and found to be 99.2% accurate [3].
All patients over the age of eighteen years who underwent cholecystectomy were included. Patients who underwent cholecystectomy for a known gallbladder cancer or as a part of another surgical procedure (e.g., pancreatoduodenectomy) were excluded. Hospitals were divided into high and low volume centres based on the number of LBDEs performed. High volume centres performed at least two LBDEs per month during the study period, while low volume centres performed less than two LBDEs per month.
Our reporting followed the robust and already prepared report of the CholeS study and the same guidelines set by STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) [6]. Most data were analysed and presented descriptively using tables, graphs and percentages for distribution. When possible, clinically relevant groups were compared using the chi-squared test to detect differences. A p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using Microsoft Excel (v2103; Microsoft, Redmond, WA, USA), GraphPad Prism (v9.3.1; San Diego, CA, USA) and IBM SPSS Statistics (ver. 23.0; IBM Corp., Armonk, NY, USA).
During the study period, 8,820 LCs were performed. There were 256 patients (2.9%) who underwent LBDE and 75 patients (0.85%) who had partially complete data sets (whether they underwent LBDE was unknown). Of a total of 166 hospitals that took part in this study, 77 hospitals (46.4%) performed at least one LBDE. In these units, 5.4% of patients who underwent LC also underwent LBDE. Almost 90% (69/77) of hospitals that performed LBDEs carried out less than or equal to five procedures during the two-month study period. Only 5.2% (4/77) of hospitals that carried out LBDE performed more than ten, accounting for 19.8% of their LC workload, compared to 2% to 8% for the remaining units. Fig. 1 categorises the included units by the number of LBDEs performed. Of the 256 patients who underwent LBDE, 173 patients (67.6%) were females. The median age of these 256 patients was 59 years (range: 15–86 yr). Most patients were either American Society of Anesthesiologists (ASA) grade I (33.2%) or ASA grade II (55.3%). Body mass index (BMI) was not recorded for individual patients. However, each patient was placed in a BMI category. Almost 40% of patients were obese (BMI > 30 kg/m2). The majority (76.5%) were either overweight or obese (BMI > 25 kg/m2).
During the study period, compared to the 256 (2.9%) LBDEs performed, 932 patients (10.6%) underwent ERCP and endoscopic sphincterotomy. Of the 256 patients who underwent LBDEs, 73 patients (28.5%) underwent preoperative ERCP and 112 patients (43.8%) underwent preoperative MRCP. Forty-four patients had both MRCP and ERCP prior to LBDE (17.2%). Of note, on preoperative imaging, 131 patients (51.2%) had a dilated bile duct (diameter > 6 mm) and 113 patients (44.1%) had a normal calibre bile duct (diameter ≤ 6 mm). Over three quarters (78.5%) underwent intraoperative cholangiogram (IOC) (Table 1). LBDEs were mainly performed by upper gastrointestinal (GI) surgeons (84.4%) and colorectal surgeons (10.0%). Eighty-seven percent and 13.0% of LBDEs were performed by consultants and trainees, respectively.
The laparoscopic-to-open conversion rate was 12.5%. The median operation time was 111 minutes (interquartile range [IQR]: 75–155 minutes). Median length of hospital stay (LOHS) was six days (IQR: 4–11 days) for LC + LBDEs performed in the acute setting, and five days (IQR: 3–8 days) for acute LC without LBDE (p < 0.01). For elective LC + LBDE, median LOHS was one day (IQR: 1–4 days), which was significantly longer than that for elective LC (0 day [IQR: 0–1 day]; p < 0.01). Postoperative hospital stay (POHS) was two days (IQR: 2–6 days) for acute LC + LCBDE and two days (IQR: 1–3 days) for acute LC (p < 0.01). Elective POHS was one day (IQR: 1–4 days) for LC + LCBDE and zero days (IQR: 0–1 day) for elective LC (p < 0.01). Among LBDE cases, overall postoperative morbidity and bile leak rates were 21.5% and 4.7%, respectively. Thirty-day readmission rate was 12.1% and 30-day mortality rate was 0.4% (Table 2).
Bile leak, acute pancreatitis, retained stone, and overall morbidity rates following LBDE were compared between low- and high-volume centres. Bile leak (odds ratio [OR]: 8.62, 95% confidence interval [CI]: 2.26–32.84, p < 0.01) and 30-day overall morbidity rate (OR: 1.90, 95% CI: 1.05–3.44, p = 0.04) were significantly higher in low-volume centres. There was no significant difference in the rate of postoperative pancreatitis (OR: 0.85, 95% CI: 0.09–8.3, p = 0.89) or retained stone rate (OR: 0.76, 95% CI: 0.16–3.68, p = 0.74).
Our results highlight that LBDEs are performed at a low rate (< 3%), despite recent evidence which suggests that LBDE is safe and feasible. Whilst LBDE is often perceived to be more invasive, ERCP is not without risk. It is known that ERCP can destroy the physiological barrier between the gut and the pancreatobiliary system [2]. Multiple reasons might be behind the low LBDE uptake. Firstly, many hospitals run a reliable ERCP service. This option might be perceived to be less invasive. Secondly, general surgical training tends to neglect LBDE and general surgical theatres often lack the appropriate equipment. Finally, theatre lists are under increasing time pressure. By utilising the option of ERCP and electing not to perform LBDE, surgeons can divert patients to another resource and free up valuable time in theatre.
Interestingly, 29% and 44% of patients who underwent LBDE also underwent failed preoperative ERCP and preoperative MRCP, respectively. As such, many patients with BDS were subjected to a long and convoluted treatment pathway. The British Society of Gastroenterology (BSG) guidelines [1] advise that either a single-stage or a two-stage approach can be performed. However, our results suggest the uptake of LBDE is considerably lower than the endoscopic treatment. Less than half of hospitals performed a single LBDE during the study period. Explorations made up 5.4% of the total cholecystectomy workload (256 out of 4,763 cholecystectomies performed). The vast majority of hospitals that performed LBDE carried out less than or equal to five procedures during the study period. A minority of procedures were carried out at high-volume centres. Results of our recent UK-wide survey (the ALiCE survey [7]) suggest that these patterns have remained. We found a heavy reliance on preoperative MRCP and a preference for the two-stage approach among surgeons [7]. We also found that 80% of surgeons would still perform MRCP in patients with abnormal liver function tests and/or ultrasound findings suggestive of BDS [7]. In addition, 62% of surgeons would opt for a two-stage approach whereas only 33% would opt for a single-stage approach (34% stated they would utilise intraoperative ultrasound and 66% stated they would perform an IOC) [7].
In the present study, upper GI surgeons performed the majority of LBDEs. As one might expect, over 50% of upper GI surgeons were oesophagogastric (OG) surgeons and approximately 30% were hepatopancreatobiliary (HPB) surgeons. This is not surprising given the greater number of OG surgeons compared to HPB surgeons across the UK and RoI. We also noted that over 86% of LBDEs were performed by consultant-level surgeons. Results from a survey of European-African Hepato-Pancreato-Biliary Association (E-AHPBA) members in 2016 suggested that 75% of LBDEs were performed by HPB surgeons and 22% were performed by non-HPB specialists [8]. The survey found 16% of cases were carried out by trainees. It was 14% in the present study. According to a recent United States of America (USA) survey conducted at two academic conferences, 77% of participants stated that they would favour ERCP over LBDE as the initial treatment of BDS [9]. At the time of questioning, participants had performed a median of zero (mean: 1, range: 0–10) LBDEs. Among those who had completed a minimally invasive fellowship, a median of zero (mean: 2, range: 0–20) LBDEs had been performed. Participants also stated that the most significant barriers to performing LBDE were “inadequate familiarity with the procedure” (mean: 3.1, scale: 1–5), “lack of equipment” (mean: 3.1, scale: 1–5), and “lack of technical ability” (mean: 2.8, scale: 1–5). It raised the question about adequate training for LBDE as a minority of procedures were performed by trainees. Our results suggest that the learning curve is carried out mostly after the completion of formal training. Thus, a greater emphasis should be placed on LBDE during upper GI surgical training.
Following a retrospective study of 390 cases where choledochotomy bile duct exploration with primary closure after LC was carried out, Zhu et al. [10] found that the learning curve for this procedure was achieved after approximately 54 cases. After that, operation time (94 min vs. 117 min, p < 0.01), overall morbidity rate (5.7% vs. 16.7%, p < 0.01), bile leak rate (1.5% vs. 7.4%, p < 0.01), and rate of retained stones (0.3% vs. 3.7%, p < 0.01) were all significantly lower [10]. A national assessment of USA trends in BDS management found a marked decline in the use of LBDE between 1998–2013, in favour of ERCP + LC [11]. The authors conclude that if the trend continues in the USA, LBDE is at risk of disappearing from surgical armamentarium. A USA nationwide inpatient sample recently found that only 7% of patients with BDS underwent LBDE, whereas 93% underwent ERCP [12].
There are no sizeable multicentre series published on outcomes of LBDE. Several single institution series have been published with patient numbers ranging from 200 to 416 [13-16]. Al-Ardah et al. [13] published a series (n = 200) from a 13-year period. POHS was 5.8 days and 30-day readmission was 6%. In our series, POHS was two days and 30-day readmission was 12.1%. Postoperative ERCP rate was 18.4% [13], compared to 7.0% in the present study. However, the series in the study of Al-Ardah et al. [13] spanned over 13 years and practice might have changed considerably during this time. Al-Ardah et al. [13] used a T-tube in 25% of patients; this could explain the longer POHS and the higher postoperative ERCP rate in their study.
Aawsaj et al. [14] published a series of 296 LBDEs performed over a five-year period and the conversion rate was 4%. In our study it was 13%. Mean postoperative stay was five days for both acute and elective cases. The median postoperative stay was one day in our series. Bile leak rate was 5% in their study [14], similar to our study (4.7%). The most likely explanation for the higher conversion rate in the present study was its multicentre design and the inclusion of both high- and low- volume centres. However, we did not evaluate the reasons for conversion or investigate the correlation between procedure volume and conversion rate [14].
Navartne and Martinez Isla [15] have published a series (n = 416) from a 10-year period. They compared outcomes between trans-cystic LBCE (TC-LBDE) and trans-ductal LBDE (TD-LBDE) [15]. Overall, bile leak rate was 3.5% [15]. Postoperative acute pancreatitis rate was 7.4% in the TD-LCBDE group and 0.6% in the TC-LCBDE group. The overall acute pancreatitis rate in their study was considerably higher than that (1.6%) in our series. This may reflect the high usage of antegrade stents (10.9 % vs. 4.0% in primary closure) in their series [15]. In a further series (n = 346), Zhang et al. [16] found 3% of LBDE patients experienced bile leak, which was slightly lower than that (5.3%) in our study. The conversion rate was 4.5% in their study. It was 12.5% in our series. The higher conversion in our series might reflect the multicentre study design which included some centres that performed less than two cases during the two-month study period. The retained stone rate in their study was 4% [16], which was comparable to that (3.1%) in our study.
It is unclear why the use of the single-stage approach for managing symptomatic gallstones was so low during the study window. Considering results of the recent ALiCE survey [7], it is likely that this pattern will remain. A large multicentre study with a longer study period is needed to investigate this. In a survey performed in 2016 asking American surgeons why the uptake of LBDE was low, Baucom et al. [17] found that common responses were: “reliable and available ERCP service”, “lack of equipment”, “lack of comfort with LBDE”, and “technically challenging/no formal training”. Similar responses were given in the UK-based ALiCE survey [7]. LBDE is a technically challenging procedure that requires the acquisition of advanced laparoscopic skills. In addition, it requires the use of instruments not commonly used by general surgeons. Furthermore, whilst recent evidence and guidelines support the single-stage approach, there is enormous variability amongst surgeons’ opinions and techniques regarding LBDE. These include variations in patient selection, type of intraoperative bile duct imaging, mode of entry into the bile duct (trans-cystic vs. trans-choledochal), type of instrument (choledochoscope vs. fluoroscopy), type of choledochotomy (vertical vs. transverse incision), method of choledochotomy closure (primary closure vs. over T-tube vs. biliary stent) and intraperitoneal drain use. The fact that there is not one optimum agreed method for performing LBDE may be a further reason for the low uptake of LBDE. Finally, surgeons may be reluctant to perform LBDE as they fear a bile duct stricture or additional morbidity. Hence, they may favour the two-stage approach, which is perceived to be less invasive.
This study has several limitations. Although the CholeS study was robust, its primary focus was on collecting data of patients undergoing LC, not LBDE. As limited data were collected regarding LBDE, we could not comment on different operative techniques used, patient selection, or variability of the procedure itself. Although we compared LC patients who underwent LBDE to those who did not (Table 2), these two groups had differing levels of risk. In addition, we were unable to consider certain confounding variables. We did not have data on complications of ERCP. We were unable to exclude LC patients who did not have BDS either. In addition, since data are now several years old, it is difficult to know how generalisable and applicable our results are. However, our own recent survey suggests that practice has not changed dramatically since the period of data collection [7]. Thus, we feel that our conclusions remain valid.
During the study period, only 2.9% of patients underwent LBDE. The majority of hospitals did not perform this procedure at all. Very few were performed in “high volume” units. Our results suggest that the single-stage approach for the management of BDS was underutilised despite the recent evidence that suggests it is a safe and reasonable approach. Our results could not provide any insight into why the uptake of LBDE was low. We suspect that there are multiple reasons for this. Large retrospective/prospective studies are needed in the future to investigate this to provide information that can further guide patient management.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.22-001.
REFERENCES
1. Williams E, Beckingham I, El Sayed G, Gurusamy K, Sturgess R, Webster G, et al. 2017; Updated guideline on the management of common bile duct stones (CBDS). Gut. 66:765–782. DOI: 10.1136/gutjnl-2016-312317. PMID: 28122906.
2. Lyu Y, Cheng Y, Li T, Cheng B, Jin X. 2019; Laparoscopic common bile duct exploration plus cholecystectomy versus endoscopic retrograde cholangiopancreatography plus laparoscopic cholecystectomy for cholecystocholedocholithiasis: a meta-analysis. Surg Endosc. 33:3275–3286. DOI: 10.1007/s00464-018-06613-w. PMID: 30511313.
3. Vohra RS, Spreadborough P, Johnstone M, Marriott P, Bhangu A, Alderson D, et al. 2015; Protocol for a multicentre, prospective, population-based cohort study of variation in practice of cholecystectomy and surgical outcomes (The CholeS study). BMJ Open. 5:e006399. DOI: 10.1136/bmjopen-2014-006399. PMID: 25582453. PMCID: PMC4298090.
4. CholeS Study Group, West Midlands Research Collaborative. 2016; Population-based cohort study of variation in the use of emergency cholecystectomy for benign gallbladder diseases. Br J Surg. 103:1716–1726. DOI: 10.1002/bjs.10288. PMID: 27748962.
5. Griffiths EA, Hodson J, Vohra RS, Marriott P, Katbeh T, Zino S, et al. 2019; Utilisation of an operative difficulty grading scale for laparoscopic cholecystectomy. Surg Endosc. 33:110–121. DOI: 10.1007/s00464-018-6281-2. PMID: 29956029. PMCID: PMC6336748.
6. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. 2007; Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 335:806–808. DOI: 10.1136/bmj.39335.541782.AD. PMID: 17947786. PMCID: PMC2034723.
7. Tanase A, Aroori S, Dhanda A, Cramp M, Streeter A. 2021; A UK survey on variation in practice of management of choledocholithiasis and laparoscopic common bile duct exploration (ALiCE Survey). HPB. 23(Suppl 3):S943–S944. DOI: 10.1016/j.hpb.2021.08.590.
8. Vannijvel M, Lesurtel M, Bouckaert W, Houben B, Knol J, Vangertruyden G, et al. 2016; A survey of European-African surgeons' management of common bile duct stones. HPB (Oxford). 18:959–964. DOI: 10.1016/j.hpb.2016.10.007. PMID: 27838253. PMCID: PMC5144544.
9. Teitelbaum E, Soper N, Patel P, Santos BF, Hungness ES. 2015. A survey to assess surgeon experience with, and barriers to performing, laparoscopic common bile duct exploration for teatment of choledocholithiasis [Internet]. Los Angeles: Society of American Gastrointestinal and Endoscopic Surgeons;Available from: https://www.sages.org/meetings/annual-meeting/abstracts-archive/a-survey-to-assess-surgeon-experience-with-and-barriers-to-performing-laparoscopic-common-bile-duct-exploration-for-teatment-of-choledocholithiasis/. cited 2022 Jan 12.
10. Zhu H, Wu L, Yuan R, Wang Y, Liao W, Lei J, et al. 2018; Learning curve for performing choledochotomy bile duct exploration with primary closure after laparoscopic cholecystectomy. Surg Endosc. 32:4263–4270. DOI: 10.1007/s00464-018-6175-3. PMID: 29602995.
11. Wandling MW, Hungness ES, Pavey ES, Stulberg JJ, Schwab B, Yang AD, et al. 2016; Nationwide assessment of trends in choledocholithiasis management in the United States from 1998 to 2013. JAMA Surg. 151:1125–1130. DOI: 10.1001/jamasurg.2016.2059. PMID: 27556900.
12. Poulose BK, Arbogast PG, Holzman MD. 2006; National analysis of in-hospital resource utilization in choledocholithiasis management using propensity scores. Surg Endosc. 20:186–190. DOI: 10.1007/s00464-005-0235-1. PMID: 16362476.
13. Al-Ardah M, Barnett RE, Morris S, Abdelrahman T, Nutt M, Boyce T, et al. 2021; Lessons learnt from the first 200 unselected consecutive cases of laparoscopic exploration of common bile duct stones at a district general hospital. Surg Endosc. 35:6268–6277. DOI: 10.1007/s00464-020-08127-w. PMID: 33140155.
14. Aawsaj Y, Light D, Horgan L. 2016; Laparoscopic common bile duct exploration: 15-year experience in a district general hospital. Surg Endosc. 30:2563–2566. DOI: 10.1007/s00464-015-4523-0. PMID: 26307600.
15. Navaratne L, Martinez Isla A. 2021; Transductal versus transcystic laparoscopic common bile duct exploration: an institutional review of over four hundred cases. Surg Endosc. 35:437–448. DOI: 10.1007/s00464-020-07522-7. PMID: 32246237.
16. Zhang WJ, Xu GF, Huang Q, Luo KL, Dong ZT, Li JM, et al. 2015; Treatment of gallbladder stone with common bile duct stones in the laparoscopic era. BMC Surg. 15:7. DOI: 10.1186/1471-2482-15-7. PMID: 25623774. PMCID: PMC4417333.
17. Baucom RB, Feurer ID, Shelton JS, Kummerow K, Holzman MD, Poulose BK. 2016; Surgeons, ERCP, and laparoscopic common bile duct exploration: do we need a standard approach for common bile duct stones? Surg Endosc. 30:414–423. DOI: 10.1007/s00464-015-4273-z. PMID: 26092008.
Fig. 1
Graph illustrating the number of laparoscopic bile duct explorations (LBDEs) performed by the included hospitals during the two-month study period. a)Outlier: one hospital performed 65 LBDEs.
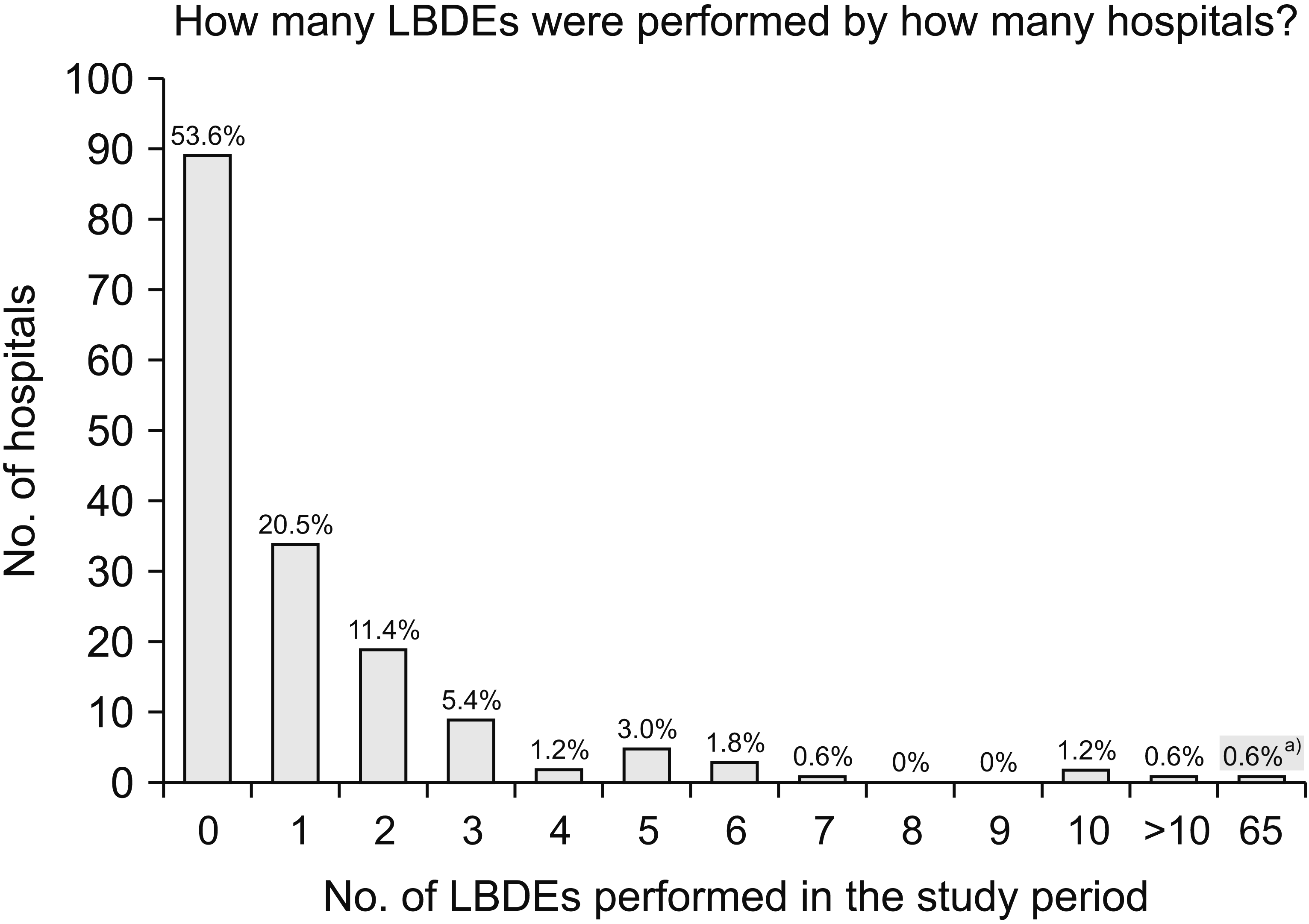
Table 1
Patient demographics and preoperative investigations
| Variable | LC + LBDE (n = 256) | All LCs (n = 8,489) (includes patients without BDS) | p-valuea) |
|---|---|---|---|
| Age (yr) | 59 (40–70) | 51 (38–64) | < 0.01*b) |
| Sex | 0.02* | ||
| Female | 173 (67.6) | 6,285 (74.0) | |
| Male | 83 (32.4) | 2,204 (26.0) | |
| BMI (kg/m2) | 0.07 | ||
| < 18 | 1 (0.4) | 38 (0.5) | |
| 18–25 | 47 (19.3) | 1,664 (20.5) | |
| 25–30 | 102 (41.8) | 2,876 (35.5) | |
| 31–35 | 49 (20.1) | 1,989 (24.5) | |
| 36–40 | 42 (17.2) | 1,509 (18.6) | |
| > 40 | 3 (1.2) | 27 (0.3) | |
| ASA | 0.04 | ||
| ASA I | 84 (33.2) | 3,263 (38.8) | |
| ASA II | 140 (55.3) | 4,295 (51.0) | |
| ASA III | 29 (11.5) | 838 (10.0) | |
| ASA IV | 0 (0.0) | 21 (0.2) | |
| ASA V | 0 (0.0) | 1 (0.0) | |
| CT | < 0.05* | ||
| No | 208 (81.3) | 7,228 (85.7) | |
| Yes | 48 (18.8) | 1,207 (14.3) | |
| MRCP | < 0.01* | ||
| No | 144 (56.3) | 6,302 (74.6) | |
| Yes | 112 (43.8) | 2,142 (25.4) | |
| Preoperative ERCP | < 0.01* | ||
| No | 183 (71.5) | 7,579 (89.9) | |
| Yes | 73 (28.5) | 854 (10.1) | |
| EUS | 0.57 | ||
| No | 254 (99.2) | 8,328 (98.8) | |
| Yes | 2 (0.8) | 98 (1.2) | |
| IOC | < 0.01* | ||
| Not performed | 55 (21.5) | 7,636 (90.0) | |
| Planned | 193 (75.4) | 755 (8.9) | |
| Unplanned | 8 (3.1) | 96 (1.1) |
Some percentages may appear incorrect as BMI was unknown in 398 patients and ASA grade was unknown in 74 patients.
LC, laparoscopic cholecystectomy; LBDE, laparoscopic bile duct exploration; BDS, bile duct stones; BMI, body mass index; ASA, American Society of Anesthesiologists; CT, computed tomography; MRCP, magnetic resonance cholangiopancreatography; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; IOC, intraoperative cholangiogram.
Table 2
Postoperative complications
| Outcome of interest | LC + LBDE (n = 256) | All LCs (n = 8,489) (includes patients without BDS) | p-valuea) |
|---|---|---|---|
| Length of hospital stay in days | |||
| Acute | 6 (4–11) | 5 (3–8) | < 0.01*b) |
| Elective | 1 (1–4) | 0 (0–1) | < 0.01*b) |
| Postoperative hospital stay in days | |||
| Acute | 2 (1–5) | 2 (1–3) | < 0.01*b) |
| Elective | 1 (1–4) | 0 (0–1) | < 0.01*b) |
| Bile leak | 12 (4.7) | 105 (1.2) | < 0.01* |
| Retained stone | 8 (3.1) | 73 (0.9) | < 0.01* |
| Pancreatitis | 4 (1.6) | 31 (0.4) | 0.02* |
| Intra-abdominal collection | 9 (3.5) | 175 (2.1) | 0.12 |
| Wound infection | 8 (3.1) | 172 (2.0) | 0.26 |
| Delayed bile duct Injury | 2 (0.8) | 5 (0.1) | 0.02* |
| Re-imaging | 46 (18.0) | 607 (7.2) | < 0.01* |
| Postoperative ERCP | 18 (7.0) | 156 (1.8) | < 0.01* |
| Re-laparoscopy | 2 (0.8) | 59 (0.7) | 0.70 |
| Re-laparotomy | 1 (0.4) | 32 (0.4) | 0.63 |
| 30-day complications | |||
| ED presentations | 31 (12.1) | 661 (7.8) | 0.02* |
| Readmissions | 31 (12.1) | 582 (6.9) | < 0.01* |
| Complications | 55 (21.5) | 876 (10.3) | < 0.01* |
| Mortality | 1 (0.4) | 9 (0.1) | 0.26 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download