INTRODUCTION
MATERIALS AND METHODS
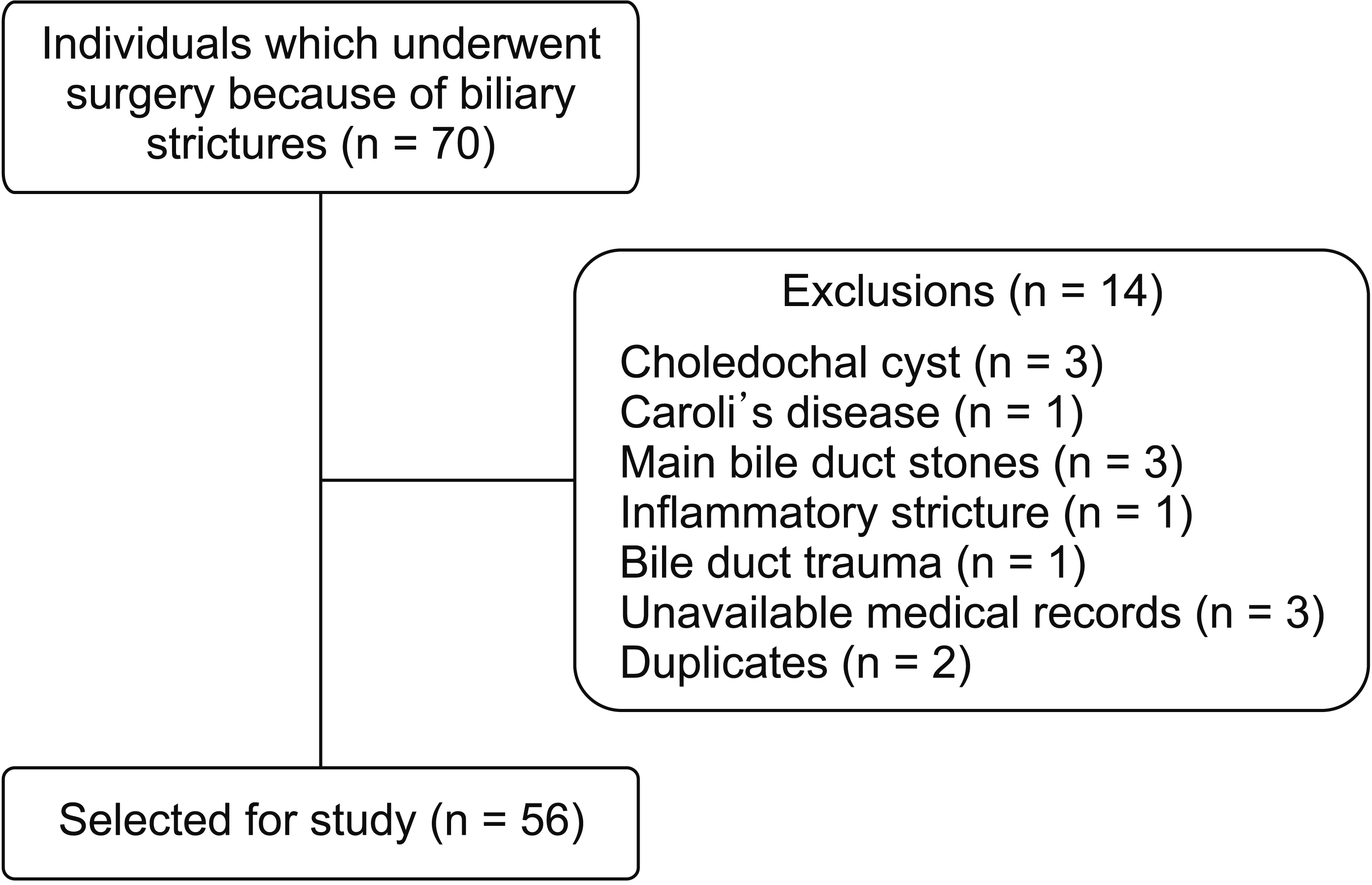
Study design
Study population
Variables
Statistical analysis
RESULTS
Table 1
Table 2
Table 3
| Variable | Hospital stay | ICU stay | Estimated bleeding | Operative time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||
| R | p-value | R | p-value | R | p-value | R | p-value | ||||
| Age | 0.2 | 0.30 | 0.2 | 0.29 | 0.07 | 0.59 | 0.07 | 0.63 | |||
| Time elapsed between cholecystectomy and repair | 0.03 | 0.82 | 0.03 | 0.82 | –0.08 | 0.64 | 0.1 | 0.38 | |||
| Aspartate aminotransferase | 0.3 | 0.06 | 0.2 | 0.18 | 0.3* | 0.05* | 0.1 | 0.32 | |||
| Alanine aminotransferase | 0.3* | 0.04* | 0.2 | 0.15 | 0.3 | 0.04 | 0.2 | 0.25 | |||
| Alkaline Phosphatase | –0.1 | 0.62 | 0.03 | 0.82 | –0.02 | 0.84 | 0.05 | 0.74 | |||
| Gamma-glutamyl transferase | –0.1 | 0.58 | 0.06 | 0.74 | –0.08 | 0.63 | 0.1 | 0.26 | |||
| Bilirrubin | –0.1 | 0.62 | 0.1 | 0.45 | –0.1 | 0.44 | –0.2 | 0.15 | |||
| International normatized ratio | 0.03 | 0.83 | 0.1 | 0.51 | –0.1 | 0.39 | –0.004 | 0.99 | |||
| Albumin | –0.1 | 0.62 | 0.1 | 0.49 | –0.06 | 0.64 | –0.04 | 0.84 | |||
| Strasberg classification degree | 0.2 | 0.09 | 0.07 | 0.64 | 0.3* | 0.02* | 0.06 | 0.62 | |||
| Body mass index | –0.1 | 0.39 | 0.1 | 0.29 | –0.02 | 0.93 | –0.3 | 0.07 | |||
| Fibrosis severity | 0.06 | 0.64 | 0.002 | > 0.99 | 0.2 | 0.18 | 0.3* | 0.03* | |||
| Cholestasis severity | 0.2 | 0.17 | 0.2 | 0.07 | 0.2 | 0.27 | –0.1 | 0.45 | |||
Table 4
| Variable | Mild to moderate complication (Clavien-Dindo 1-2) | Severe complication (Clavien-Dindo ≥ 3) | p-value | Late stricture | No stricture | p-value |
|---|---|---|---|---|---|---|
| Number | 42 (75.0) | 14 (25.0) | NA | 12 (21.4) | 44 (78.6) | NA |
| Age (yr) | 46.3 ± 13.0 | 50.0 ± 13.9 | 0.39 | 41.3 ± 12.5 | 48.8 ± 13.0 | 0.08 |
| Sex |
M: 11 (26.2) F: 31 (73.8) |
M: 2 (14.3) F: 12 (85.7) |
0.42 |
M: 2 (16.7) F: 10 (83.3) |
M: 11 (25.0) F: 33 (75.0) |
0.49 |
| Body mass index (kg/m2) | 26.8 ± 5.1 | 25.9 ± 4.9 | 0.58 | 27.1 ± 5.3 | 25.7 ± 4.7 | 0.72 |
| Time elapsed between cholecystectomy and repair (mon) | 29.58 ± 43.91 | 22.6 ± 27.0 | 0.56 | 27.58 ± 39.45 | 29.66 ± 40.50 | 0.88 |
| Aspartate aminotransferase (U/L) | 52.2 ± 36.7 | 72.9 ± 45.9 | 0.10 | 73.0 ± 57.3 | 66.3 ± 40.9 | 0.62 |
| Alanine aminotransferase (U/L) | 69.3 ± 57.4 | 103.4 ± 78.4 | 0.12 | 91.6 ± 48.5 | 95.7 ± 80.9 | 0.87 |
| Alkaline phosphatase(U/L) | 506.1 ± 595.9 | 433.4 ± 455.0 | 0.63 | 799.5 ± 832.6 | 402.9 ± 307.0 | 0.01* |
| Gamma-glutamyl transferase (U/L) | 693.5 ± 856.0 | 697.1 ± 488.8 | 0.97 | 792.7 ± 853.4 | 667.6 ± 509.5 | 0.46 |
| Bilirrubin (mg/dL) | 5.8 ± 8.1 | 6.0 ± 6.8 | 0.92 | 7.7 ± 7.8 | 5.4 ± 7.8 | 0.35 |
| International normatized ratio | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.48 | 1.1 ± 0.2 | 1.1 ± 0.1 | > 0.99 |
| Albumin (g/dL) | 3.4 ± 0.8 | 3.8 ± 0.5 | 0.04* | 3.5 ± 0.7 | 3.8 ± 0.6 | 0.09 |
| Strasberg E1-E2 vs. ≥ E3 |
1–2: 22 ≥ 3: 20 |
1–2: 7 ≥ 3: 7 |
0.88 |
1–2: 6 (50.0) ≥ 3: 6 (50.0) |
1–2: 22 (50.0) ≥ 3: 22 (50.0) |
> 0.99 |
| Fibrosis |
Present: 37 (88.1) Ausente: 5 (11.9) |
Present: 12 (85.7) Absent: 2 (14.3) |
0.79 |
Present: 11 (91.7) Absent: 1 (8.3) |
Present: 38 (86.4) Absent: 6 (13.6) |
0.62 |
| Cholestasis |
Present: 28 (66.7) Absent: 14 (33.3) |
Present: 7 (50.0) Absent: 7 (50.0) |
0.27 |
Present: 8 (66.7) Absent: 4 (33.3) |
Present: 27 (61.4) Absent: 17 (38.6) |
0.68 |
| Bile culture |
Positive: 20 (47.6) Negative: 22 (52.4) |
Positive: 10 (71.5) Negative: 4 (28.5) |
0.23 |
Positive: 7 (58.3) Negative: 5 (41.7) |
Positive: 23 (52.3) Negative: 21 (47.7) |
0.68 |
Table 5
| Variable | Hospital stay | ICU stay | Estimated bleeding | Operative time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||
| R | p-value | R | p-value | R | p-value | R | p-value | ||||
| Age | –0.12 | 0.52 | –0.12 | 0.53 | 0.08 | 0.65 | –0.21 | 0.21 | |||
| Body mass index | –0.20 | 0.21 | –0.25 | 0.12 | –0.04 | 0.76 | –0.23 | 0.09 | |||
| Strasberg classification | 0.01 | 0.93 | –0.18 | 0.27 | 0.31* | < 0.05* | 0.16 | 0.23 | |||
| AST | –0.26 | 0.40 | –0.41 | 0.19 | 0.10 | 0.73 | 0.17 | 0.50 | |||
| ALT | –0.09 | 0.73 | 0.16 | 0.55 | –0.33 | 0.19 | –0.33 | 0.15 | |||
| ALP | 0.20 | 0.37 | 0.18 | 0.41 | 0.13 | 0.54 | 0.008 | 0.96 | |||
| GGT | 0.02 | 0.90 | –0.14 | 0.49 | 0.04 | 0.82 | 0.17 | 0.31 | |||
| Totak bilirubin | 0.02 | 0.93 | 0.28 | 0.34 | –0.3 | 0.28 | –0.36 | 0.15 | |||
| Fibrosis severity | 0.09 | 0.56 | 0.23 | 0.16 | –0.09 | 0.57 | 0.4* | 0.02* | |||
| Steatosis severity | 0.21 | 0.19 | –0.02 | 0.89 | 0.3 | 0.05 | 0.10 | 0.44 | |||
| Acute cholangitis | 0.009 | 0.96 | 0.12 | 0.48 | –0.04 | 0.77 | 0.01 | 0.91 | |||
| Chronic cholangitis | –0.09 | 0.56 | –0.05 | 0.72 | 0.07 | 0.63 | 0.3* | 0.02* | |||
| Cholestasis | –0.12 | 0.62 | –0.43 | 0.10 | 0.18 | 0.46 | 0.11 | 0.61 | |||
| Ductular proliferation | 0.01 | 0.92 | 0.09 | 0.61 | 0.02 | 0.86 | –0.02 | 0.88 | |||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download