Abstract
Backgrounds/Aims
Gallstone disease is a recognized complication of bariatric surgery. Subsequent management of choledocholithiasis may be challenging due to altered anatomy which may include Roux-en-Y gastric bypass (RYGB). We conducted a retrospective service evaluation study to assess the safety and efficacy of endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE) in patients with RYGB anatomy.
Methods
All the patients who underwent EDGE for endoscopic retrograde cholangiopancreatography after RYGB at two tertiary care centers in the United Kingdom between January 2020 and October 2021 were included in the study. Clinical and demographic details were recorded for all patients. The primary outcome measures were technical and clinical success. Adverse events were recorded. Hot Axios lumen apposing metal stents measuring 20 mm in diameter and 10 mm in length were used in all the patients for creation of a gastro-gastric or gastro-jejunal fistula.
Results
A total of 14 patients underwent EDGE during the study period. The majority of the patients were female (85.7%) and the mean age of patients was 65.8 ± 9.8 years. Technical success was achieved in all but one patient at the first attempt (92.8%) and clinical success was achieved in 100% of the patients. Complications arose in 3 patients with 1 patient experiencing persistent fistula and weight gain.
Obesity is a major public health problem across the globe. In the UK, approximately, two thirds of the adult population are overweight and half of these are obese [1]. Bariatric surgery is an effective treatment modality for patients who are morbidly obese (body mass index [BMI] > 40 kg/m2) or with BMI 35–39.9 kg/m2 with complications such as diabetes, osteoarthritis, hypertension and dyslipidaemia [2]. Roux-en-Y gastric bypass (RYGB) is a common and highly successful surgical obesity treatment; a recent systemic review and meta-analysis showed that laparoscopic RYGB is more efficacious than laparoscopic sleeve gastrectomy even in elderly obese individuals, further endorsing the technique [3]. However, it is associated with comorbidities.
Symptomatic gall stone disease may develop after bariatric surgery either because of the underlying obesity or due to rapid post-surgical weight loss [4-6]. Endoscopic retrograde cholangiopancreatography (ERCP) after RYGB is often technically challenging due to altered anatomy. Device (enteroscopy) assisted (EA)-ERCP after RYGB, e.g., double balloon enteroscopy, single balloon enteroscopy or spiral enteroscopy have very low success rates and are seldom attempted in current practice. Consequently, alternative access methods such as laparoscopy-assisted ERCP (LA-ERCP), gastrostomy assisted ERCP or the recently popularized endoscopic ultrasound (EUS)-directed transgastric ERCP (EDGE) are becoming more common. LA-ERCP has a high technical success rate but conveys a higher risk and cost. A recent meta-analysis comparing device assisted ERCP (DA-ERCP) and LA-ERCP reported a higher success rate for LA-ERCP, but at a higher cost, procedural time and adverse event rate [7]. Laparoscopic common bile duct exploration (LCBDE) is another treatment approach for biliary events following RYGB. Fuente et al. [8] reported their experience of Laparoscopic trans-cystic bile duct exploration (LTCBDE) in patients who developed biliary events after RYGB. The authors compared the outcomes between two groups; one which underwent LTCBDE along with laparoscopic cholecystectomy and one without LTCBDE. Length of hospital stay and morbidity did not differ between the two groups.
EDGE involves creating a passage between the remnant and excluded stomach using a lumen apposing metal stent (LAMS) through which the duodenoscope can subsequently be passed. It has potential advantages over LA-ERCP and EA-ERCP in terms of clinical and technical success, procedural times and adverse events, but it is currently only performed at a few centers owing to a requirement for expertise in EUS and LAMS deployment. A recent multicentric experience of clinical outcomes between LA-ERCP and EDGE demonstrated similar technical success and adverse events, although EDGE had an advantage as far as procedure time and length of hospital stay are concerned. EDGE may offer a minimally invasive, effective option, with less resource utilization, and reassuringly without causing significant weight gain [9]. We conducted this dual center retrospective observational study to present the early experience of EDGE in patients with RYGB from the United Kingdom.
We conducted a two-center, retrospective review as part of a service evaluation of consecutive patients who underwent EDGE at two institutions in the United Kingdom (The Leeds Teaching Hospitals NHS Trust and University Hospital Southampton NHS Foundation Trust) between January 2020 and October 2021. Data on demographics, baseline patient characteristics, procedural information and post-procedural events were obtained from each institution’s electronic records database.
The procedures were done under general anaesthesia by therapeutic endoscopists. Prophylactic antibiotics were administered following each institution’s policy (Fig. 1, Supplementary Video).
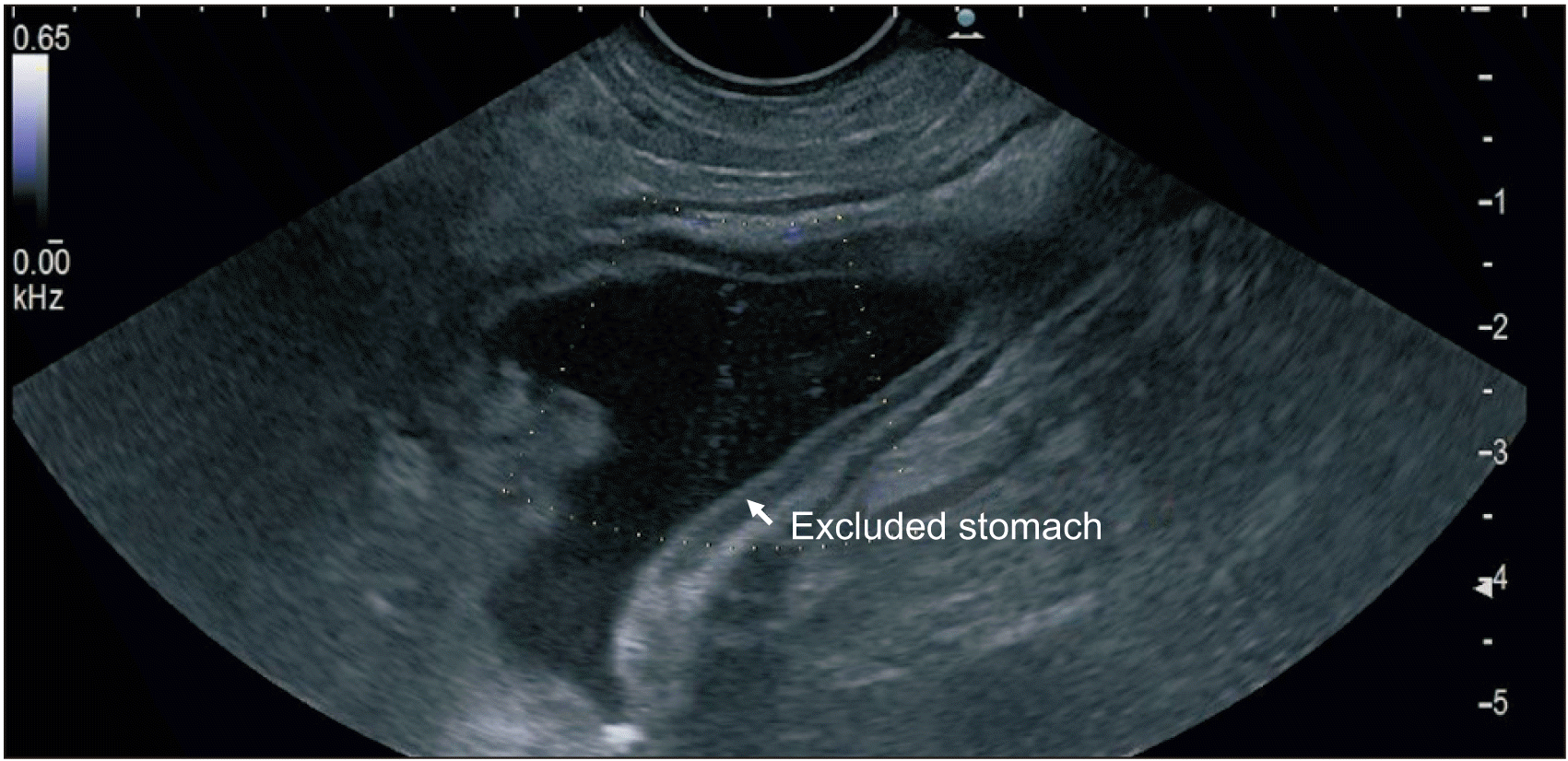
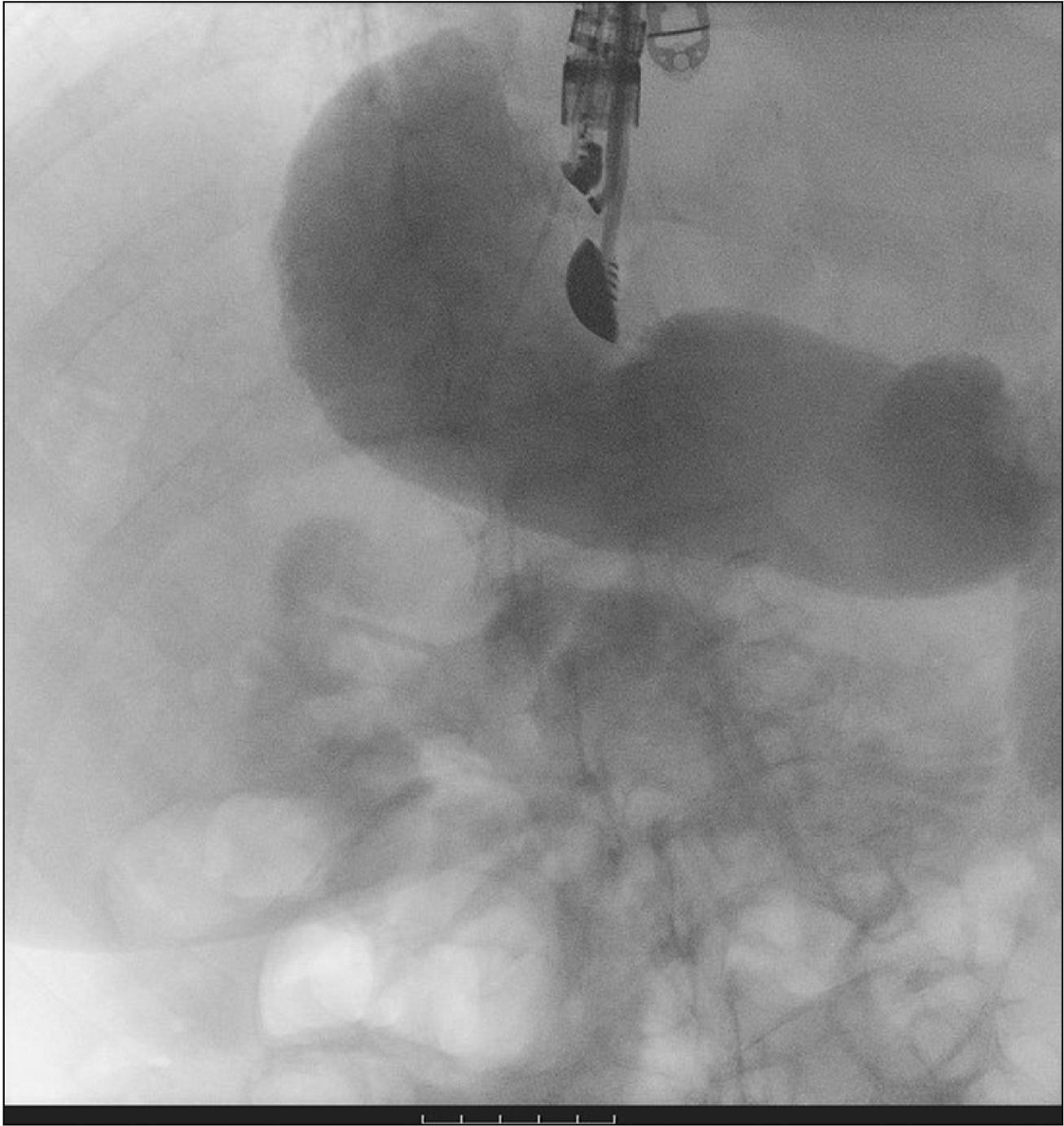
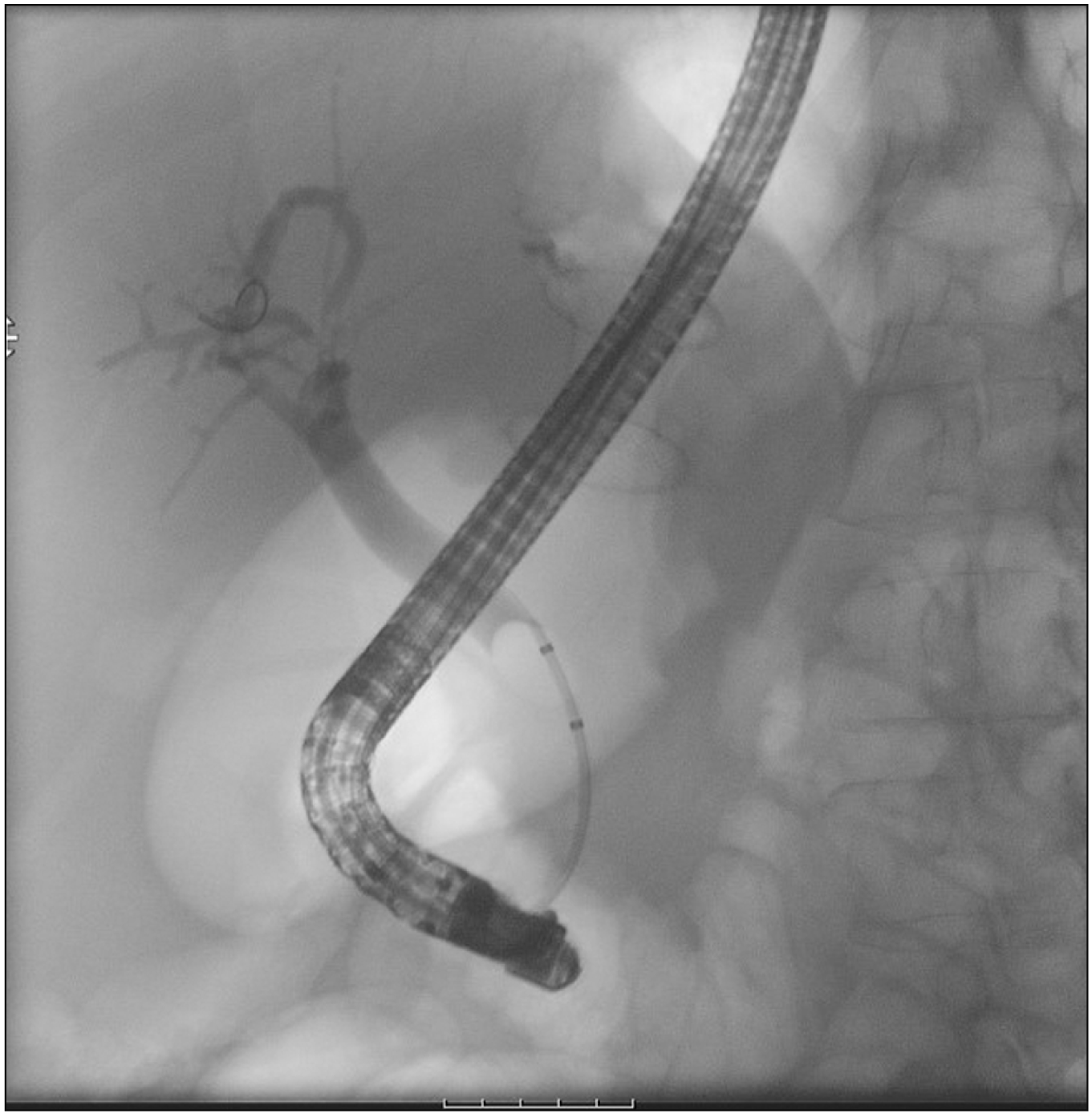
A therapeutic linear echo-endoscope was used to identify the excluded stomach (Fig. 2), either from the remnant stomach or the proximal Roux limb. The excluded stomach was punctured using a 19-gauge fine needle biopsy needle (Fig. 3). Diluted contrast was injected under fluoroscopy (Fig. 4) and EUS to confirm the correct position of the needle before further contrast/saline was instilled to distend the stomach and delineate the distal anatomy. The choice of gastro-gastric or jejuno-gastric puncture was at the discretion of the performing endoscopist. After direct puncture of the excluded stomach using the electrocautery tip (without using a guidewire to assist in stent insertion), the delivery catheter was advanced into the stomach and the distal flange of the LAMS was deployed under EUS guidance (Fig. 5). The proximal flange was released under EUS or endoscopic view. For single session EDGE, the stent lumen was dilated with a controlled radial expansion balloon up to 18–20 mm. For two stage EDGE, stent dilatation was allowed to occur gradually during the interval between the procedures.
ERCP (Fig. 6) was either performed in the same setting as LAMS deployment or as a second stage procedure. The interval period in case of second stage ERCP was at the discretion of the endoscopist but was planned within a 4 weeks of LAMS placement. LAMS removal was planned within 8 weeks.
After removal of LAMS, no attempt was made to close the fistula unless the patient had weight gain or evidence of persistent fistula. Fistula closure was confirmed with gastroscopy after 4 weeks and then a barium meal was done at 12 weeks to assess for persistent fistula.
The primary outcome measures were technical and clinical success. Secondary outcome measures were adverse events. Technical success was defined as the ability to perform a conventional ERCP with deep biliary cannulation following insertion of the LAMS. Clinical success was defined as the normalization of bilirubin and/or the resolution of the patient’s symptoms.
A total of 14 patients underwent EDGE during the evaluation period. The mean age of the patients was 65.8 ± 9.8 years, and the majority were female (85.7%). Eleven patients (78.6%) had previously undergone cholecystectomy (Table 1, 2).
All the patients had undergone RYGB for morbid obesity. In 12 out of the 14 patients who required EDGE, the indication for ERCP was choledocholithiasis only. One patient had a bile leak post cholecystectomy in addition to choledocholithiasis. The other patient had stenosis of the ampulla of Vater.
EUS guided LAMS was deployed across the gastro-gastric site in 11 (78.6%) and jejuno-gastric site in 3 (21.4%) patients. Technical success was achieved in 13 patients (92.8%) on the first attempt. One patient required a second attempt on day 3 as the first attempt led to stent mis-deployment leading to pneumo-peritoneum and a fully covered oesophageal stent was placed to bridge the defect. The procedure was successful on the second attempt. A 20 mm LAMS was used in all the patients. Eight patients (57.1%) underwent balloon dilatation of the LAMS after stent deployment; 18 mm balloon in 6 and 20 mm balloon in 2.
A single session combined EUS + ERCP was done in 8 patients (57.1%). In the other 6 patients (42.9%), ERCP was performed as a second stage procedure after a median period of 10.5 days (8–13 days). Clinical success was achieved in all the patients. The mean indwelling LAMS time in 12 patients was 53.2 ± 41.8 days (median 43 days). One patient had retrograde stent migration into the residual stomach and one had her stent removed elsewhere at an unspecified time.
Adverse events were seen in 3 patients (21.4%). In the first patient, pneumoperitoneum was noted on the fluoroscopic image following completion of ERCP and withdrawal of the duodenoscope. Computed tomography on day 3 showed migration of LAMS into the excluded stomach, but no contrast extravasation, and the patient remained asymptomatic. The second patient developed a persistent fistula after removal of the LAMS and started gaining weight. Endoscopic suturing of the fistula was performed, but barium meal confirmed persistent fistula. A second procedure for endoscopic suturing was performed and a follow-up barium meal is anticipated. The third patient developed pneumoperitoneum after the initial LAMS mis-deployment and a fully covered 8 cm (length) × 18 mm (width) oesophageal stent was placed to bridge the defect successfully. The patient did not develop any clinical sequelae and 3 days later underwent a successful ERCP with the passage of the duodenoscope through the indwelling oesophageal stent.
We present the first experience of EDGE after RYGB surgery in the UK, highlighting the technical feasibility, clinical efficacy and safety profile of the procedure.
A systemic review of DA-ERCP after RYGB showed an overall success rate of 70% for ERCP using DA-ERCP after RYGB [10] and it remains popular in some settings due to lower costs and difficulty accessing surgery [11] or EDGE. However, it is associated with a low technical success rate which means that a significant number of patients cannot be helped.
LA-ERCP has a higher success rate than DA-ERCP and is beneficial if the patient needs a simultaneous cholecystectomy. However, it carries risks associated with additional surgery, long procedural times and higher costs [12-16]. A multicentric study comparing EDGE/GATE (gastric access temporary for endoscopy) and LA-ERCP showed that the clinical and technical success and adverse events were comparable between the two techniques, however, EDGE/GATE had a short post procedure hospitalization and procedure time [9]. James et al. [17] conducted a cost effectiveness analysis of EDGE vs. DA-ERCP and LA-ERCP in patients with RYGB anatomy and concluded that, among the three, EDGE was the most cost-effective technique for the treatment of pancreaticobiliary disorders in patients with RYGB anatomy. A recent meta-analysis demonstrated that EDGE has a higher technical and clinical success rate compared to DA-ERCP and is comparable to LA-ERCP. Notably, the adverse events were similar to LA-ERCP but higher than DA-ERCP [18].
LCBDE in patients with RYGB [19] is a well established procedure for single stage management of choledocholithiasis. However, there are many barriers to the widespread adoption of LCBDE, including insufficient training and lack of exposure to LCBDE. Therefore, it should be considered alongside EDGE in this group of patients taking into account the pros and cons of both modalities and expertise available.
Recently, Barclay et al. [20] reported their experience on EDGE from Canada. They performed EDGE in 7 patients post RYGB surgery. The indication for ERCP was choledocholithiasis in 6 patients and gall stone pancreatitis in 1 patient, respectively. Twenty mm LAMS were used in all the patients and all the patients underwent an ERCP as a second stage procedure with a median interval of 9 days between LAMS insertion and ERCP. Double pigtail plastic stents were inserted after LAMS removal and were removed at a later date to allow staged fistula closure. The technical and clinical success was 100% and no adverse events were reported. Runge et al. [21] reported their multicenter experience of EDGE across 13 centers (12 US and 1 European). A total of 178 patients underwent EDGE and technical success was achieved in 175 patients. Half of the patients underwent LAMS deployment across the gastro-gastric site (50.2%) and half through the gastro-jejunal site (49.7%). In 63% of the cases, 15 mm diameter LAMS was used and 20 mm was used in the remaining 37%, of the cases in contrast to our study where 20 mm LAMS was deployed universally. LAMS were removed in 153 patients after a median duration of 35 days which is comparable to the LAMS indwelling time in our cohort. There were 28 (15.7%) early adverse events which included perforation, intra-procedural migration of LAMS, mis-deployment of LAMS, post procedure bleeding, post ERCP pancreatitis, cholangitis and delayed LAMS migration. Long term complications in the form of persistent fistula were identified in 9 out of the 90 patients. Five out of these 9 patients underwent successful endoscopic closure of fistulae and 3 out of the 9 patients had weight gain during the study follow up.
Tyberg et al. [22] reported their single center experience of consecutive patients undergoing EDGE by a single operator. A total of 19 patients underwent EDGE in this study. Indications for EDGE included symptomatic biliary stricture, choledocholithiasis and pancreatitis. Two patients underwent jejunogastric LAMS while the other patients had gastro-gastric LAMS. Technical success was 100% and clinical success was achieved in all but one patient. The patient in whom clinical success could not be achieved had a jejunal perforation while advancing the duodenoscope through the LAMS. ERCP was performed in the same session in 5 out of the 19 patients while the rest of the patients underwent ERCP at an interval of 2 to 3 weeks. Four patients experienced complications in the study cohort; two had bleeding, one had post ERCP pancreatitis, and one had jejunal perforation. Runge et al. [21] described management of persistence fistula after LAMS removal. The authors concluded that post-EDGE fistulae are easily manageable owing to endoscopic closure devices and are clinic-patho-physiologically different from other gastro-gastric and gastro-jejunal fistulas. With further studies and experience, the EDGE technique will get refined, clear protocols will be developed for one stage vs. two stage procedures and post LAMS removal monitoring of fistulae and weight gain.
Our study limitations include the retrospective nature of the evaluation, a relatively small number of patients, lack of a dedicated protocol on the timing of ERCP (single session vs. interval) and variability in the juncture at which the LAMS was removed due to logistical issues secondary to the ongoing pandemic. The strengths of our study include a nearly 100% technical and a 100% clinical success rate across two large centers.
In conclusion, our series and the work of others provide significant evidence of the safety and efficacy of the EDGE procedure. We propose it as the appropriate access technique for patients requiring biliary access following RYGB surgery. We recognize that variation in institutional skills and limited experience in EUS and LAMS training, may limit its application globally.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.22-019.
REFERENCES
1. NHS Digital. Statistics on obesity, physical activity and diet: England, 2020. Leeds: NHS Digital;2020.
2. Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. 2014; The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 149:275–287. DOI: 10.1001/jamasurg.2013.3654. PMID: 24352617. PMCID: PMC3962512.
3. Shenoy SS, Gilliam A, Mehanna A, Kanakala V, Bussa G, Gill T, et al. 2020; Laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass in elderly bariatric patients: safety and efficacy-a systematic review and meta-analysis. Obes Surg. 30:4467–4473. DOI: 10.1007/s11695-020-04819-3. PMID: 32594469.
4. Stender S, Nordestgaard BG, Tybjaerg-Hansen A. 2013; Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a Mendelian randomization study. Hepatology. 58:2133–2141. DOI: 10.1002/hep.26563. PMID: 23775818.
5. Shiffman ML, Sugerman HJ, Kellum JM, Brewer WH, Moore EW. 1991; Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 86:1000–1005.
6. Li VK, Pulido N, Fajnwaks P, Szomstein S, Rosenthal R, Martinez-Duartez P. 2009; Predictors of gallstone formation after bariatric surgery: a multivariate analysis of risk factors comparing gastric bypass, gastric banding, and sleeve gastrectomy. Surg Endosc. 23:1640–1644. Erratum in: Surg Endosc 2009;23:1645. DOI: 10.1007/s00464-008-0204-6. PMID: 19057954.
7. Ayoub F, Brar TS, Banerjee D, Abbas AM, Wang Y, Yang D, et al. 2020; Laparoscopy-assisted versus enteroscopy-assisted endoscopic retrograde cholangiopancreatography (ERCP) in Roux-en-Y gastric bypass: a meta-analysis. Endosc Int Open. 8:E423–E436. DOI: 10.1055/a-1070-9132. PMID: 32118116. PMCID: PMC7035133.
8. Fuente I, Beskow A, Wright F, Uad P, de Santibañes M, Palavecino M, et al. 2021; Laparoscopic transcystic common bile duct exploration as treatment for choledocholithiasis after Roux-en-Y gastric bypass. Surg Endosc. 35:6913–6920. DOI: 10.1007/s00464-020-08201-3. PMID: 33398581.
9. Kedia P, Tarnasky PR, Nieto J, Steele SL, Siddiqui A, Xu MM, et al. 2019; EUS-directed transgastric ERCP (EDGE) versus laparoscopy-assisted ERCP (LA-ERCP) for Roux-en-Y gastric bypass (RYGB) anatomy: a multicenter early comparative experience of clinical outcomes. J Clin Gastroenterol. 53:304–308. DOI: 10.1097/MCG.0000000000001037. PMID: 29668560.
10. Skinner M, Popa D, Neumann H, Wilcox CM, Mönkemüller K. 2014; ERCP with the overtube-assisted enteroscopy technique: a systematic review. Endoscopy. 46:560–572. DOI: 10.1055/s-0034-1365698. PMID: 24839188.
11. Schreiner MA, Chang L, Gluck M, Irani S, Gan SI, Brandabur JJ, et al. 2012; Laparoscopy-assisted versus balloon enteroscopy-assisted ERCP in bariatric post-Roux-en-Y gastric bypass patients. Gastrointest Endosc. 75:748–756. DOI: 10.1016/j.gie.2011.11.019. PMID: 22301340.
12. Banerjee N, Parepally M, Byrne TK, Pullatt RC, Coté GA, Elmunzer BJ. 2017; Systematic review of transgastric ERCP in Roux-en-Y gastric bypass patients. Surg Obes Relat Dis. 13:1236–1242. DOI: 10.1016/j.soard.2017.02.005. PMID: 28336200.
13. Abbas AM, Strong AT, Diehl DL, Brauer BC, Lee IH, Burbridge R, et al. 2018; Multicenter evaluation of the clinical utility of laparoscopy-assisted ERCP in patients with Roux-en-Y gastric bypass. Gastrointest Endosc. 87:1031–1039. DOI: 10.1016/j.gie.2017.10.044. PMID: 29129525.
14. Grimes KL, Maciel VH, Mata W, Arevalo G, Singh K, Arregui ME. 2015; Complications of laparoscopic transgastric ERCP in patients with Roux-en-Y gastric bypass. Surg Endosc. 29:1753–1759. DOI: 10.1007/s00464-014-3901-3. PMID: 25318366.
15. May D, Vogels E, Parker D, Petrick A, Diehl D, Gabrielsen J. 2019; Overall outcomes of laparoscopic-assisted ERCP after Roux-en-Y gastric bypass and sphincter of Oddi dysfunction subgroup analysis. Endosc Int Open. 7:E1276–E1280. DOI: 10.1055/a-0832-1898. PMID: 31579709. PMCID: PMC6773570.
16. Yang D, Draganov PV. 2019; ERCP in patients with Roux-en-Y gastric bypass: one size does not fit all. Gastrointest Endosc. 89:646. DOI: 10.1016/j.gie.2018.09.014. PMID: 30784505.
17. James HJ, James TW, Wheeler SB, Spencer JC, Baron TH. 2019; Cost-effectiveness of endoscopic ultrasound-directed transgastric ERCP compared with device-assisted and laparoscopic-assisted ERCP in patients with Roux-en-Y anatomy. Endoscopy. 51:1051–1058. DOI: 10.1055/a-0938-3918. PMID: 31242509.
18. Dhindsa BS, Dhaliwal A, Mohan BP, Mashiana HS, Girotra M, Singh S, et al. 2020; EDGE in Roux-en-Y gastric bypass: how does it compare to laparoscopy-assisted and balloon enteroscopy ERCP: a systematic review and meta-analysis. Endosc Int Open. 8:E163–E171. DOI: 10.1055/a-1067-4411. PMID: 32010749. PMCID: PMC6976316.
19. Tucker O, Soriano I, Szomstein S, Rosenthal R. 2008; Management of choledocholithiasis after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 4:674–678. DOI: 10.1016/j.soard.2008.01.014. PMID: 18539541.
20. Barclay RL, Jain A, Ferland ASB, Chen YI, Donnellan F. 2022; Living on the EDGE: Canadian experience with EUS-directed transgastric ERCP (EDGE) in patients with roux-en-Y gastric bypass anatomy. J Can Assoc Gastroenterol. 5:116–120. DOI: 10.1093/jcag/gwab035. PMID: 35669842. PMCID: PMC9157288.
21. Runge TM, Chiang AL, Kowalski TE, James TW, Baron TH, Nieto J, et al. 2021; Endoscopic ultrasound-directed transgastric ERCP (EDGE): a retrospective multicenter study. Endoscopy. 53:611–618. DOI: 10.1055/a-1254-3942. PMID: 32882722.
22. Tyberg A, Kedia P, Tawadros A, Tarnasky PR, Gaidhane M, Nieto J, et al. 2020; EUS-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE): the first learning curve. J Clin Gastroenterol. 54:569–572. DOI: 10.1097/MCG.0000000000001326. PMID: 32149820.
Fig. 1
A pictorial representation of EDGE. EDGE, endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography.
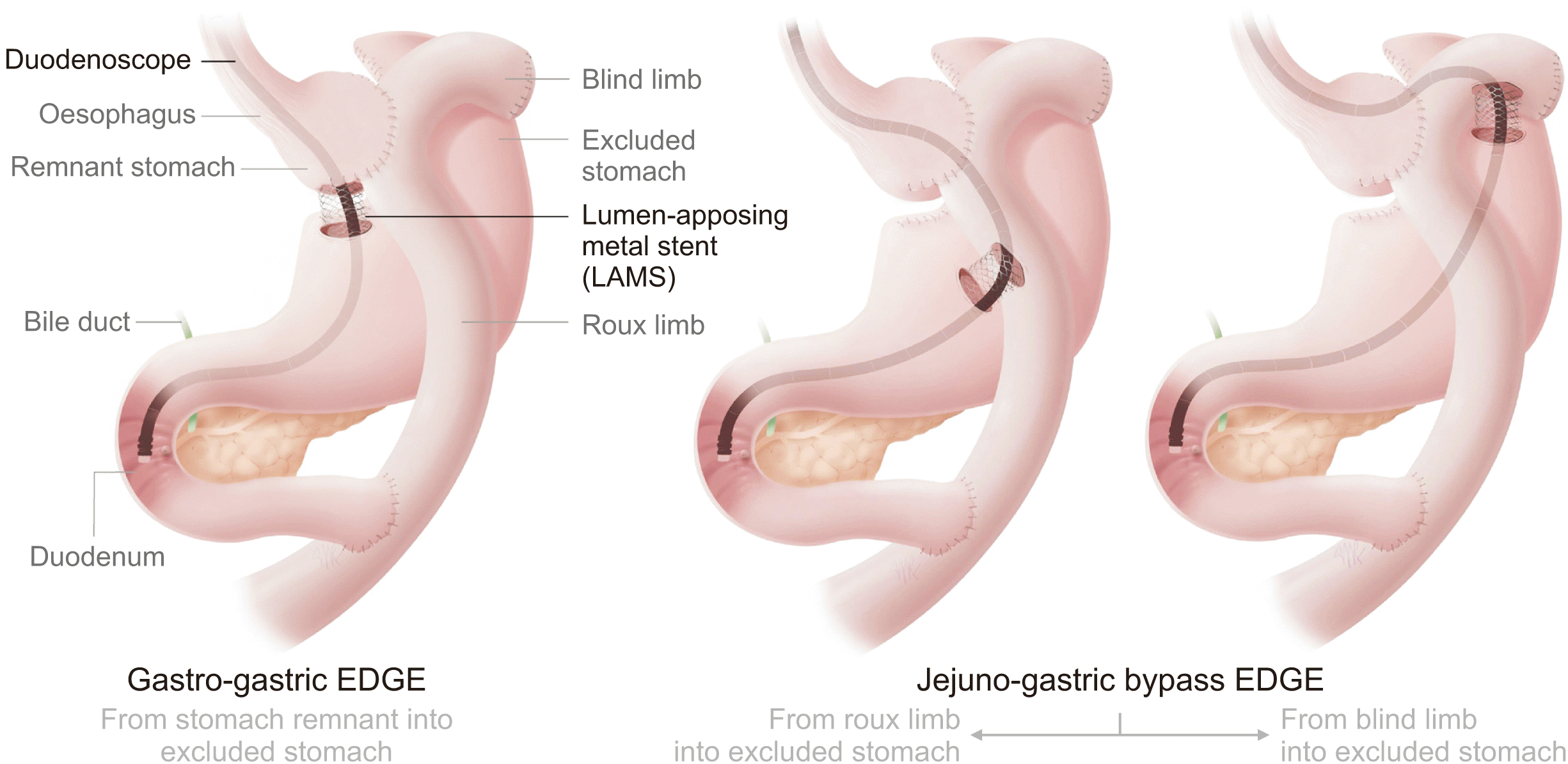
Fig. 3
Endoscopic ultrasound (EUS) image showing EUS-guided 19G needle puncture into the cavity of the excluded stomach.
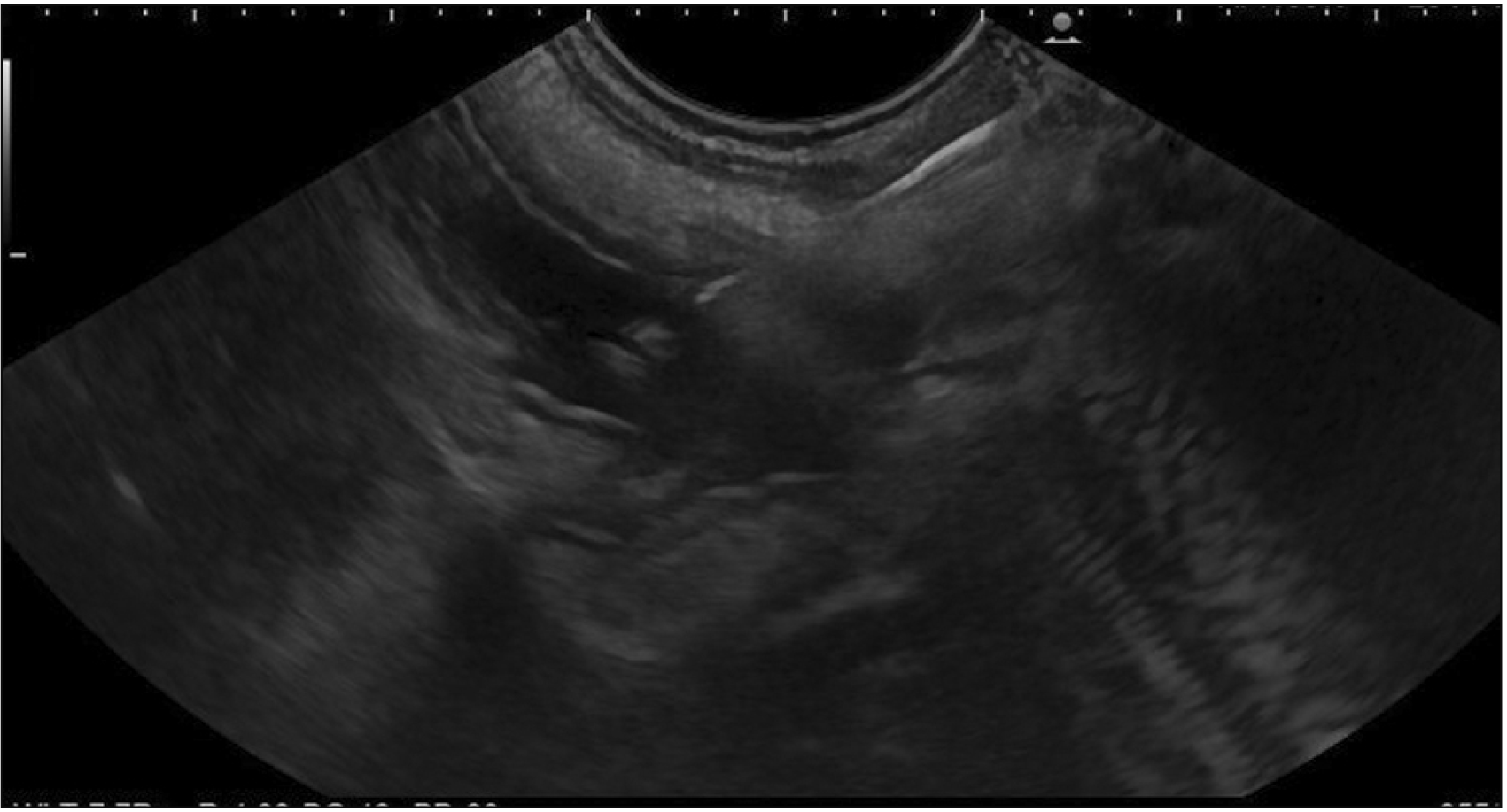
Fig. 5
Endoscopic ultrasound image showing deployment of the distal flange of lumen apposing metal stent into the residual stomach.
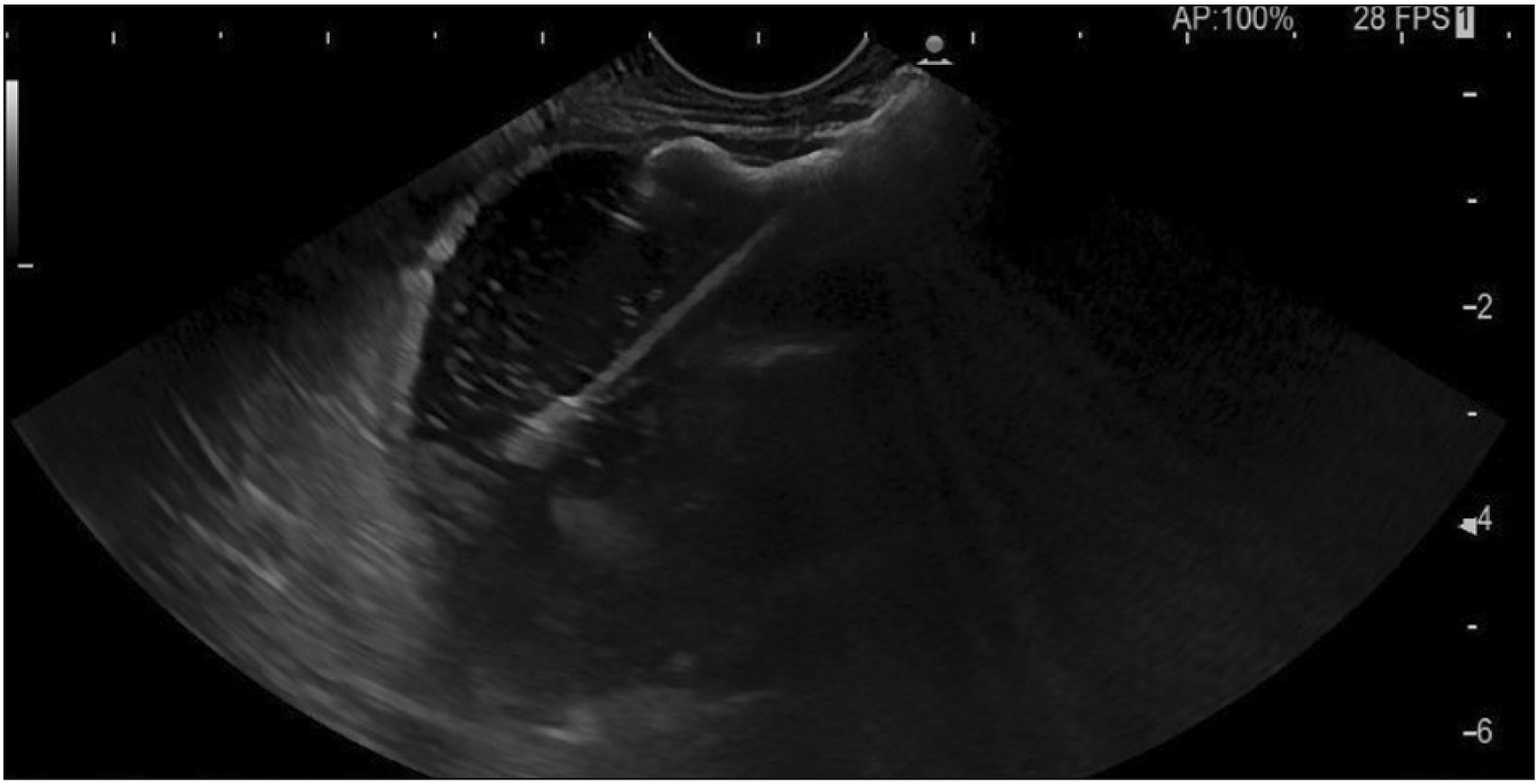
Table 1
Clinico-demographic and procedural details




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download