Abstract
Backgrounds/Aims
Although body surface area (BSA)-based standard liver volume (SLV) formulae have been used for living donor liver transplantation and hepatic resection, hemi-liver volume (HLV) is needed more frequently. HLV can be assessed using right or left portal vein diameter (RPVD or LPVD). The aim of this study was to validate the reliability of using portal vein diameter ratio (PVDR) for assessing HLV in living liver donors.
Methods
This study included 92 living liver donors (59 males and 33 females) who underwent surgery between January 2020 and December 2020. Computed tomography (CT) images were used for measurements.
Results
Mean age of donors was 35.5 ± 7.2 years. CT volumetry-measured total liver volume (TLV), right HLV, left HLV, and percentage of right HLV in TLV were 1,442.9 ± 314.2 mL, 931.5 ± 206.4 mL, 551.4 ± 126.5 mL, and 64.6% ± 3.6%, respectively. RPVD, LPVD, and main portal vein diameter were 12.2 ± 1.5 mm, 10.0 ± 1.3 mm, and 15.3 ± 1.7 mm, respectively (corresponding square values: 149.9 ± 36.9 mm2, 101.5 ± 25.2 mm2, and 237.2 ± 52.2 mm2, respectively). The sum of RPVD2 and LPVD2 was 251.1 ± 56.9 mm2. BSA-based SLV was 1,279.5 ± 188.7 mL (error rate: 9.1% ± 14.4%). SLV formula- and PVDR-based right HLV was 760.0 ± 130.7 mL (error rate: 16.2% ± 13.3%).
Living donor liver transplantation (LDLT) has been accepted as an inevitable option for management of patients with end-stage liver failure in countries with donor shortage. Provision of an adequately-sized liver graft mass is essential for successful LDLT. The use of small-for-size grafts with an excessively low graft-to-recipient weight ratio is closely associated with a decrease in graft survival [1]. Thus, accurate preoperative estimate of the graft mass is important to prevent small-for-size syndrome in LDLT recipients. Liver computed tomography (CT) is included as one of the routine preoperative imaging studies for living donors [1,2]. The gold standard for hemi-liver graft volume is direct measurement of each hemi-liver volume (HLV) using CT volumetry with manual or semiautomatic techniques [1-6]. Such type of liver volumetric measurement is a time-consuming procedure. Considering that portal blood flow into the hemi-liver is correlated with the HLV [7-9], it is possible to estimate volumetric proportions of hemi-livers using right portal vein diameter (RPVD) and left portal vein diameter (LPVD). The aim of this study was to validate the reliability of portal vein diameter comparison method for assessing HLVs in living liver donors.
This was a retrospective study with a prospective performance of CT volumetry. After reviewing our institutional database for LDLT, 92 living donors who underwent either right or left hepatectomy from January 2020 to December 2020 were randomly selected for this study. The Institutional Review Board of Asan Medical Center approved this study protocol (approval number: 2021-0857) and waived the requirement for obtaining informed consent due to the retrospective nature of this study. This study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013.
Total liver volume (TLV), right HLV, and left HLV were measured through manual CT volumetry using 5 mm-thick dynamic CT images. CT images were stored in a Picture Archiving and Communication System (PACS; Petavision3; Asan Medical Center, Seoul, Korea), enabling image processing and various measurements, including liver volumetry and area measurement. Reconstructed CT portography was used to measure maximal diameters of the main and hemi-liver portal veins. Oblique, coronal, and sagittal views of reconstructed images were used to measure portal vein diameters. RPVD and LPVD were estimated at the main portal vein bifurcation. Maximal portal vein diameters were selected to minimize potential measurement bias. These measurement processes were performed by two surgeons (SMK and AHA) majoring in hepatobiliary surgery and liver transplantation.
Body surface area (BSA) was calculated using the Mosteller formula: BSA = [body weight (kg) × height (cm) / 3,600]0.5 [10]. Standard liver volume (SLV) was calculated using the following institutional formula: SLV (mL) = –456.3 + 969.8 × BSA (m2) [11].
Portal vein diameter ratio (PVDR) was calculated using maximal RPVD and LPVD values (mm) on CT as follows: PVDR = RPVD2 / (RPVD2 + LPVD2) [7]. The RPVD to the main portal vein diameter (MPVD) ratio (MPVDR) was calculated as follows: MPVDR = RPVD2 / MPVD2. Error rate was expressed as follows: error rate (%) = {[CT volumetric HLV (mL) – PVDR-based HLV (mL)] / CT volumetric HLV (mL)]} × 100.
Continuous numeric variables are expressed as mean with standard deviation and 95% confidence interval (CI) or median with range. Continuous variables were compared with Student’s t-test. Simple linear regression analysis was performed to obtain the regression equation, correlation coefficient (r), and coefficient of determination (R2). Spearman correlation coefficient (ρ [rho]) was used for correlation analysis. A p-value < 0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY, USA) and MedCalc version 20.010 (MedCalc, Ostend, Belgium).
Demographic and anthropometric profiles of 92 living donors are summarized in Table 1. There were 59 (64.1%) male and 33 (35.9%) female donors. Their mean age was 35.5 ± 7.2 years.
Mean values of the CT volumetry-measured TLV, right HLV, left HLV, and percentage of right HLV to TLV were 1,442.9 ± 314.2 mL, 931.5 ± 206.4 mL, 551.4 ± 126.5 mL, and 64.6% ± 3.6%, respectively. Mean RPVD, LPVD, and MPVD were 12.2 ± 1.5 mm, 10.0 ± 1.3 mm, and 15.3 ± 1.7 mm, respectively. Their corresponding square values were 149.9 ± 36.9 mm2, 101.5 ± 25.2 mm2, and 237.2 ± 52.2 mm2, respectively. The sum of RPVD2 and LPVD2 was 251.1 ± 56.9 mm2, which was not statistically different from MPVD2 (p = 0.087).
The Spearman correlation coefficient ρ was 0.123 (p = 0.241) between the ratio of right HLV to TLV (right HLV ratio) and the ratio of RPVD2 to MPVD2 (MPVDR). It was 0.295 (p = 0.005) between the right HLV ratio and the ratio of RPVD2 to the sum of RPVD2 and LPVD2 (PVDR). The mean PVDR was 59.3% ± 5.2% (Table 1). Because PVDR showed a higher correlation than MPVDR, this parameter was used in further analysis of the present study. Correlation between the right HLV ratio and PVDR is depicted in Fig. 1, in which r was 0.35 and R2 was 0.12 (p < 0.001), showing the following equation: right HLV ratio = 0.244 × PVDR + 50.1.
Mean values of CT volumetry-measured TLV and formula-derived SLV were 1,442.9 ± 314.2 mL and 1,279.5 ± 188.7 mL, respectively (p < 0.001; Fig. 2). The error rate of formula-derived SLV compared with CT volumetry-measured TLV was 9.1% ± 14.4% (95% CI, 8.0%–11.4%).
Mean values of CT volumetric right HLV and SLV formula-PVDR-based right HLV were 931.5 ± 206.4 mL and 760.0 ± 130.7 mL, respectively (p < 0.001).
The correlation of right HLV ratio and PVDR is depicted in Fig. 3, in which r was 0.67 and R2 was 0.45 (p < 0.001), showing the following equation: right HLV ratio = 1.027 × PVDR + 144.8. The error rate of the SLV-based formula and PVDR-derived right HLV was 16.2% ± 13.3% (95% CI, 13.4%–18.9%).
Results of the present study showed that the combination of BSA-based SLV formula and PVDR could predict the right HLV with an error rate of 16.2% ± 13.3%. Considering that the error rate of the formula-derived SLV was 9.1% ± 14.4%, it appeared to be reliably accurate for use in clinical practice [12]. This estimation method can be theoretically used for preoperative living donor evaluation. However, due to recent technical advancement in three-dimensional volumetric measurement [3-6], there is no specific reason to use such a rough estimation in clinical practice for LDLT. A high correlation between CT-volumetric HLV and actual hemi-liver graft volume/weight has already been demonstrated [3].
On the other hand, because the PVDR can reliably reflect the proportion of each HLV to TLV, this estimation method can be applied as a part of an initial donor screening process requiring rough estimation of the size of hemi-liver graft. It can also be applied to fields where preoperative CT volumetry is unavailable. For example, it can be used for split liver transplantation, in which preoperative abdominal CT is not routinely performed. Body weight and height combined with preoperative or intraoperative ultrasonographic measurement of the RPVD and LPVD can provide a rough estimation of right and left HLVs. In a short Taiwanese study that estimated the hemi-liver graft weight for split liver transplantation with portal vein diameters derived from bedside abdominal ultrasonography, the discrepancy between the calculated hemi-liver graft weight and the actual graft weight was less than 4% [13].
The rationale for HLV proportion estimation using the PVDR was based on the observation that the sizes of right and left portal veins seemed to radiologically reflect differential hepatic masses of two hemi-livers. This concept was proposed in a Taiwanese study [7], in which sizes of the two hemi-livers were in parallel with the volume of blood flow and hepatotrophic factors in the two portal veins. Sizes of the two portal veins and their blood flow were proportional to metabolic and nutritional demands of hemi-livers. This concept is indirectly supported by some earlier animal studies, suggesting that the volume of the portal blood flow is the most important factor for liver regeneration [8,14].
On comparing BSA-based SLV with CT volumetric TLV, the error rate of formula-derived SLV was 9.1% ± 14.4%, showing a statistically significant difference. In our previous study with multiple SLV formulae that had been validated to show the lowest error ratios [15-18], comparison between formula-based SLV and volumetric TLV showed error rates greater than 10% regardless of the SLV formula type [10]. Even when the SLV formula was validated with a native mother cohort, the error rate was 10.5%. This was primarily due to the innate wide variability of individual TLVs. Although individual TLVs were influenced by sex and body mass index, compensation for sex and body mass index did not decrease the range of error rates [11]. In the present study, the combination of formula-derived SLV and PVDR increased the error rate to 16.2% ± 13.3% for estimating the right HLV. Considering these inevitable covariates of SLV, such error rates in assessing HLV seemed to be acceptable. An error rate less than 20% was considered to indicate an acceptable actual graft volume/weight estimate [12].
BSA-based SLV and error ratio of HLV secondary to SLV are inevitably influenced by the SLV formulae. The world-first formula was proposed by Urata et al. [19] in 1995. The second formula was proposed by our team as follows: SLV (mL) = 691 × BSA (m2) + 95 [20,21]. We also proposed the third formula [11], which was used in the present study. To date, the number of available formulae for SLV is more than 20 [11,18]. New formulae are continuously being proposed worldwide [22,23].
The concept of PVDR-based HLV proportion is also useful for estimating the remnant left liver volume during hepatobiliary surgery requiring right hepatectomy. Some malignant tumor invades the right liver and right portal vein, resulting in decreased RPVD with a compensatory increase of LPVD. Huge hepatocellular carcinoma occupying the right liver can also induce similar changes in the hilar portal vein system. Preoperative measurement of HLVs and confirmation through PVDR estimation would be helpful to calculate future remnant liver volume and hepatic parenchymal resection rate [11].
This study has a limitation that is worthy of noting. This was a single-center study with a relatively small number of samples. Further validation studies with a large number of healthy individuals are necessary to obtain more reliable results.
In conclusion, the combination of BSA-based SLV and PVDR appears to be a simple estimation method that can predict right or left HLV in living donor or split liver transplantation.
REFERENCES
1. Hwang S, Lee SG, Lee YJ, Sung KB, Park KM, Kim KH, et al. 2006; Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 12:920–927. DOI: 10.1002/lt.20734. PMID: 16721780.
2. Vernuccio F, Whitney SA, Ravindra K, Marin D. 2021; CT and MR imaging evaluation of living liver donors. Abdom Radiol (NY). 46:17–28. DOI: 10.1007/s00261-019-02385-6. PMID: 31901101.
3. Kwon HJ, Kim KW, Jang JK, Lee J, Song GW, Lee SG. 2020; Reproducibility and reliability of computed tomography volumetry in estimation of the right-lobe graft weight in adult-to-adult living donor liver transplantation: Cantlie's line vs portal vein territorialization. J Hepatobiliary Pancreat Sci. 27:541–547. DOI: 10.1002/jhbp.749. PMID: 32353894.
4. Lim MC, Tan CH, Cai J, Zheng J, Kow AW. 2014; CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol. 69:887–895. DOI: 10.1016/j.crad.2013.12.021. PMID: 24824973.
5. Yang X, Yang JD, Yu HC, Choi Y, Yang K, Lee TB, et al. 2018; Dr. Liver: a preoperative planning system of liver graft volumetry for living donor liver transplantation. Comput Methods Programs Biomed. 158:11–19. DOI: 10.1016/j.cmpb.2018.01.024. PMID: 29544776.
6. Mochizuki K, Takatsuki M, Soyama A, Hidaka M, Obatake M, Eguchi S. 2012; The usefulness of a high-speed 3D-image analysis system in pediatric living donor liver transplantation. Ann Transplant. 17:31–34. DOI: 10.12659/AOT.882633. PMID: 22466906.
7. Wang F, Pan KT, Chu SY, Chan KM, Chou HS, Wu TJ, et al. 2011; Preoperative estimation of the liver graft weight in adult right lobe living donor liver transplantation using maximal portal vein diameters. Liver Transpl. 17:373–380. DOI: 10.1002/lt.22274. PMID: 21445920.
8. Takeshige K, Kuroda H, Fukaya Y, Suzuki H, Hasegawa M, Yamamoto S. 1982; The role of portal blood factors in regeneration of the liver. World J Surg. 6:603–609. DOI: 10.1007/BF01657876. PMID: 7135989.
9. Tongyoo A, Pomfret EA, Pomposelli JJ. 2012; Accurate estimation of living donor right hemi-liver volume from portal vein diameter measurement and standard liver volume calculation. Am J Transplant. 12:1229–1239. DOI: 10.1111/j.1600-6143.2011.03909.x. PMID: 22221803.
10. Mosteller RD. 1987; Simplified calculation of body-surface area. N Engl J Med. 317:1098. DOI: 10.1056/NEJM198710223171717. PMID: 3657876.
11. Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, Moon DB, et al. 2015; Quantified risk assessment for major hepatectomy via the indocyanine green clearance rate and liver volumetry combined with standard liver volume. J Gastrointest Surg. 19:1305–1314. DOI: 10.1007/s11605-015-2846-8. PMID: 25947549.
12. Gondolesi GE, Yoshizumi T, Bodian C, Kim-Schluger L, Schiano T, Fishbein T, et al. 2004; Accurate method for clinical assessment of right lobe liver weight in adult living-related liver transplant. Transplant Proc. 36:1429–1433. DOI: 10.1016/j.transproceed.2004.04.094. PMID: 15251351.
13. Lee WC, Lee CS, Soong RS, Lee CF, Wu TJ, Chou HS, et al. 2011; Split liver transplantation in adults: preoperative estimation of the weight of right and left hemiliver grafts. Liver Transpl. 17:93–94. DOI: 10.1002/lt.22213. PMID: 21254350.
14. Sgro JC, Charters C, Chandler JG, Grambort DE, Orloff MJ. 1973; Site of origin of the hepatotrophic portal blood factor involved in liver regeneration. Surg Forum. 24:377–379.
15. Pomposelli JJ, Tongyoo A, Wald C, Pomfret EA. 2012; Variability of standard liver volume estimation versus software-assisted total liver volume measurement. Liver Transpl. 18:1083–1092. DOI: 10.1002/lt.23461. PMID: 22532341.
16. Hashimoto T, Sugawara Y, Tamura S, Hasegawa K, Kishi Y, Kokudo N, et al. 2006; Estimation of standard liver volume in Japanese living liver donors. J Gastroenterol Hepatol. 21:1710–1713. DOI: 10.1111/j.1440-1746.2006.04433.x. PMID: 16984594.
17. Yuan D, Lu T, Wei YG, Li B, Yan LN, Zeng Y, et al. 2008; Estimation of standard liver volume for liver transplantation in the Chinese population. Transplant Proc. 40:3536–3540. DOI: 10.1016/j.transproceed.2008.07.135. PMID: 19100432.
18. Poovathumkadavil A, Leung KF, Al Ghamdi HM, Othman Iel H, Meshikhes AW. 2010; Standard formula for liver volume in Middle Eastern Arabic adults. Transplant Proc. 42:3600–3605. DOI: 10.1016/j.transproceed.2010.07.098. PMID: 21094823.
19. Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, et al. 1995; Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 21:1317–1321. DOI: 10.1002/hep.1840210515. PMID: 7737637.
20. Hwang S, Lee SG, Lee YJ, Park KM, Jeon HB, Kim PN, et al. 1997; Calculation of standard liver volume of Korean adults. Korean J Hepatobiliary Pancreat Surg. 1:59–65.
21. Lee SG, Park KM, Hwang S, Lee YJ, Kim KH, Ahn CS, et al. 2002; Adult-to-adult living donor liver transplantation at the Asan Medical Center, Korea. Asian J Surg. 25:277–284. DOI: 10.1016/S1015-9584(09)60192-5. PMID: 12470999.
22. Feng LM, Wang PQ, Yu H, Chen RT, Wang J, Sheng X, et al. 2017; New formula for predicting standard liver volume in Chinese adults. World J Gastroenterol. 23:4968–4977. DOI: 10.3748/wjg.v23.i27.4968. PMID: 28785151. PMCID: PMC5526767.
23. Yang G, Hwang S, Song GW, Jung DH. 2021; Comparison of skeletal muscle index-based formula and body surface area-based formula for calculating standard liver volume. Ann Hepatobiliary Pancreat Surg. 25:192–197. DOI: 10.14701/ahbps.2021.25.2.192. PMID: 34053921. PMCID: PMC8180406.
Fig. 1
A scatter plot showing the correlation between portal vein diameter ratio (PVDR) and percentage of volumetric right hemi-liver volume (HLV) in total liver volume.
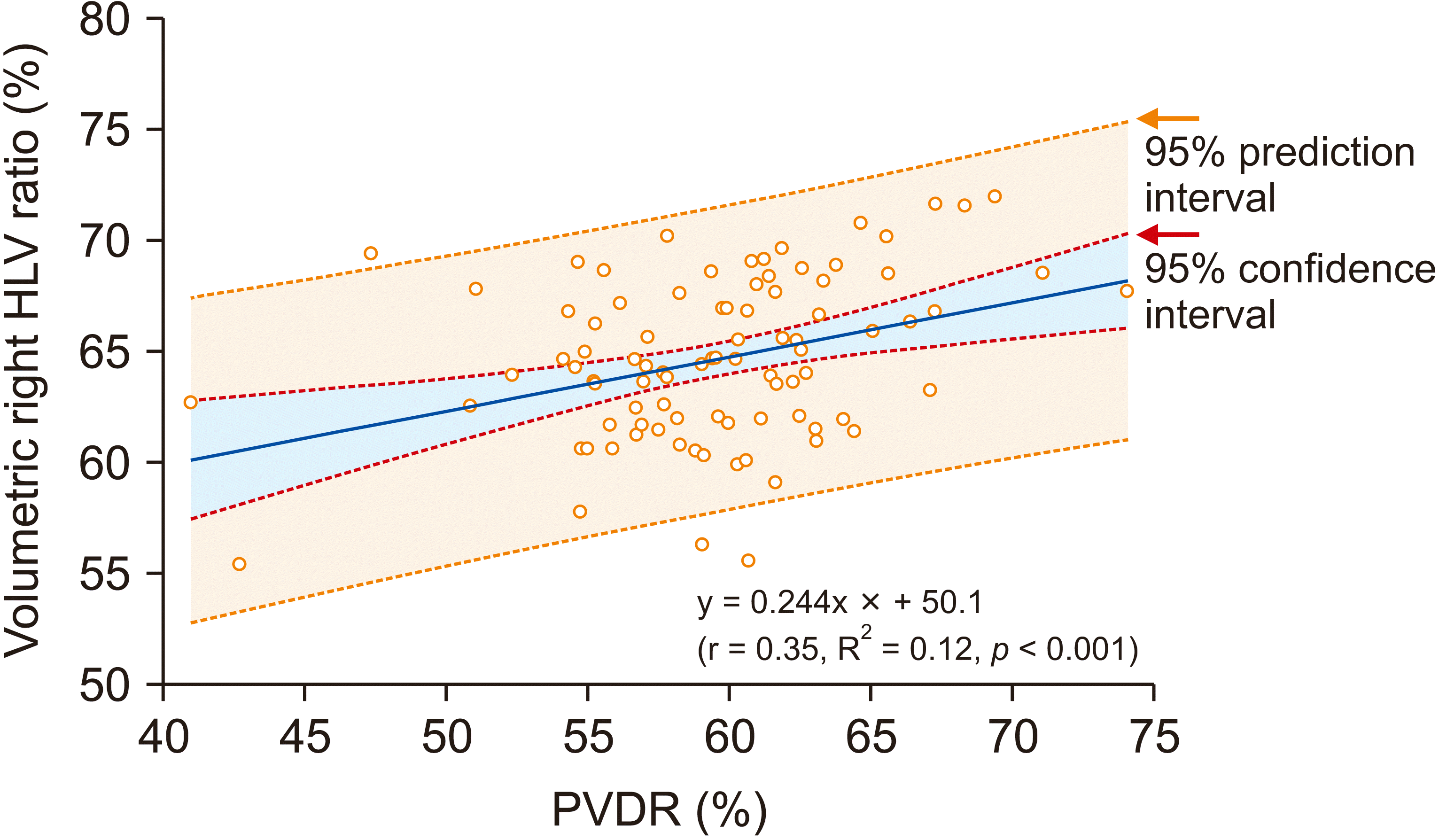
Fig. 2
Comparison of total liver volume estimated by actual computed tomography (CT) volumetry and body surface area (BSA)-based formula for standard liver volume. Boxes indicate the range of standard deviation.
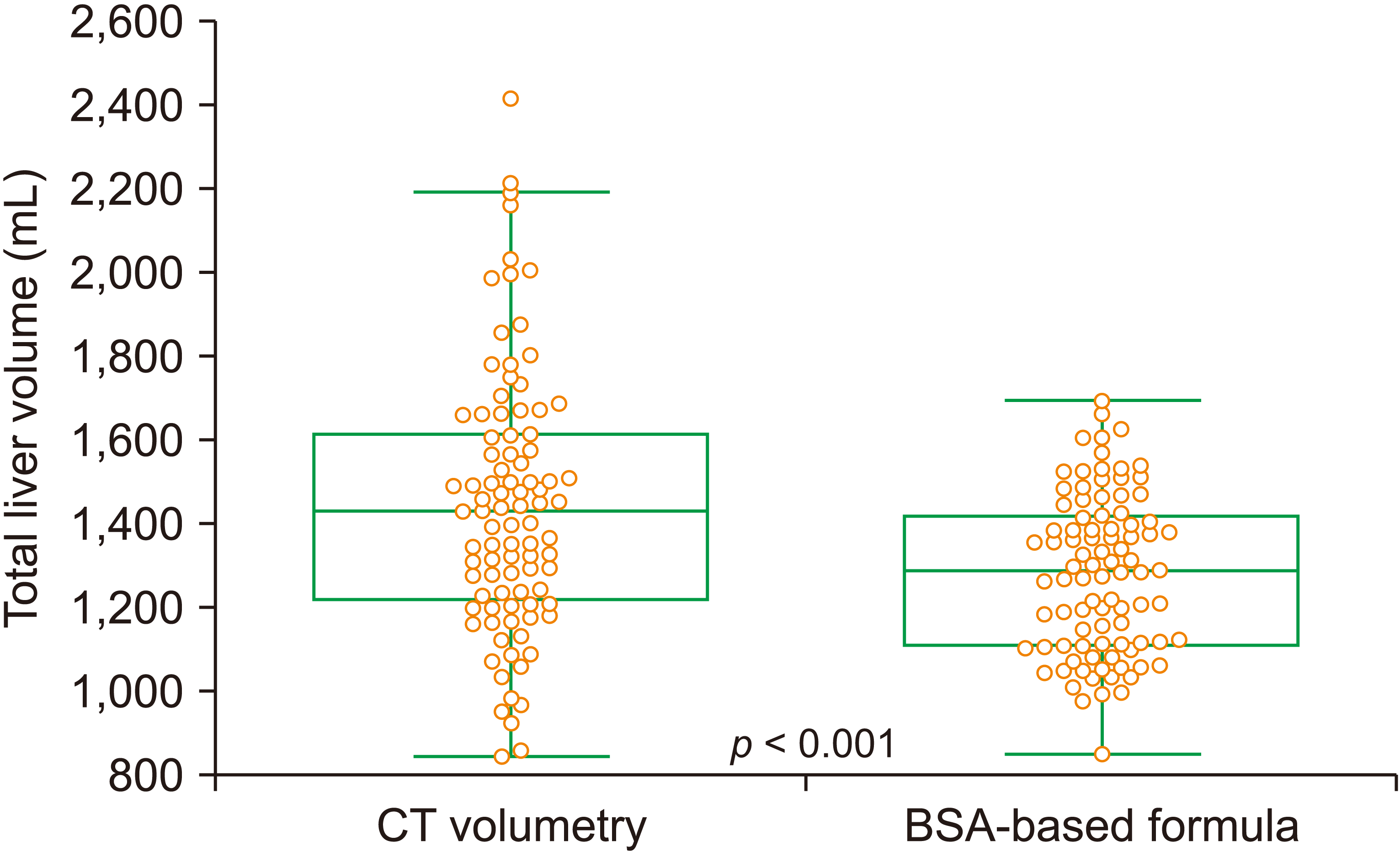
Fig. 3
A scatter plot showing correlation between right hemi-liver volume (HLV) estimated by combining standard liver volume (SLV) and portal vein diameter ratio (PVDR) and volumetric right HLV.
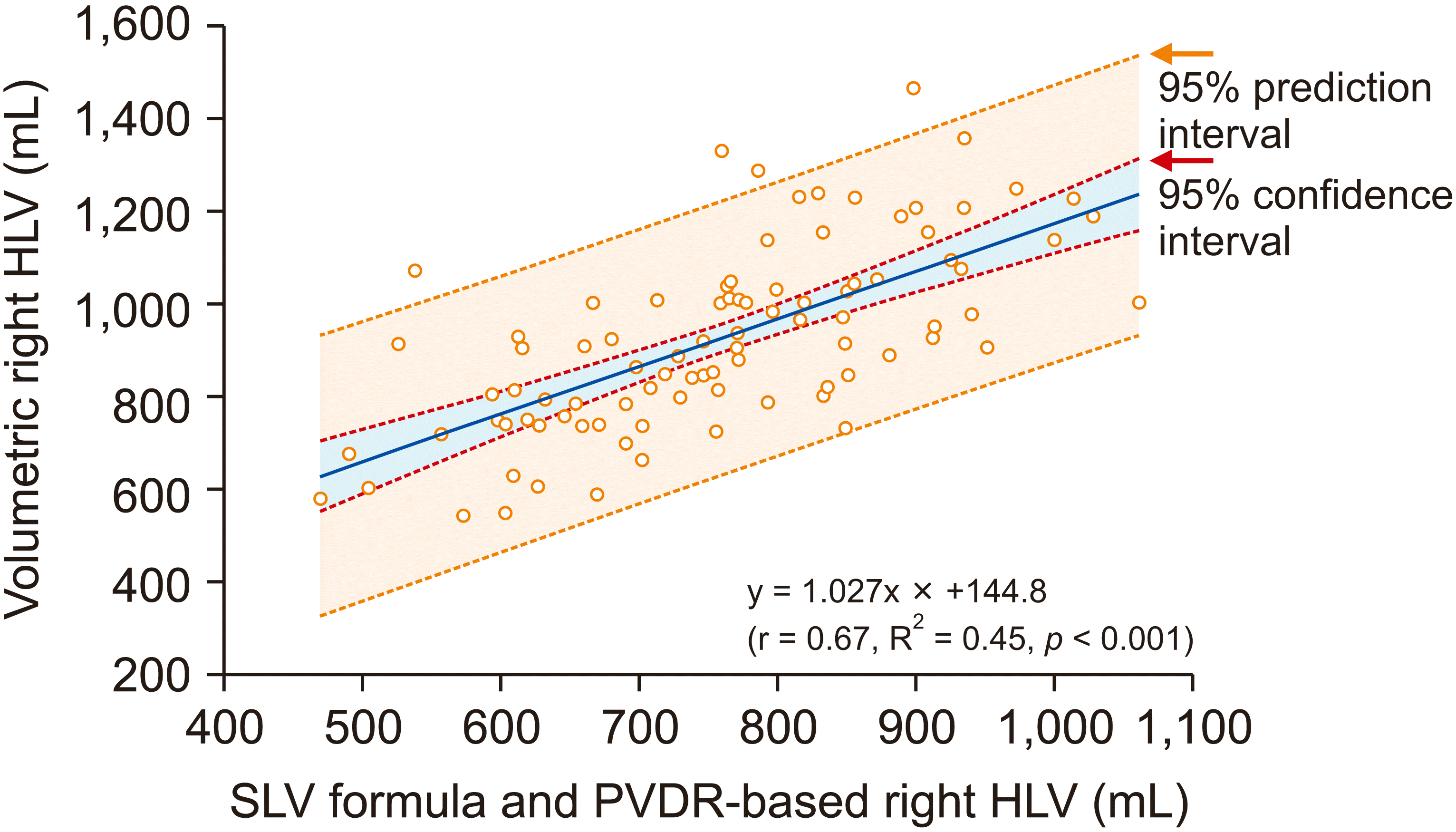
Table 1
Demographic and anthropometric profiles of 92 living liver donors




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download