Introduction
Materials and Methods
hUC-MSCs isolation and culture
Characterization of hUC-MSCs
Animal studies
Lung tissues and bronchoalveolar lavage fluid (BALF) processing
Histological analysis of lung tissues
TUNEL analysis
VEGF ELISA analysis
Western blot analysis
Measurement of pro-inflammatory cytokines by real-time PCR and ELISA
Statistical analysis
Results
Isolation and characterization of hUC-MSCs
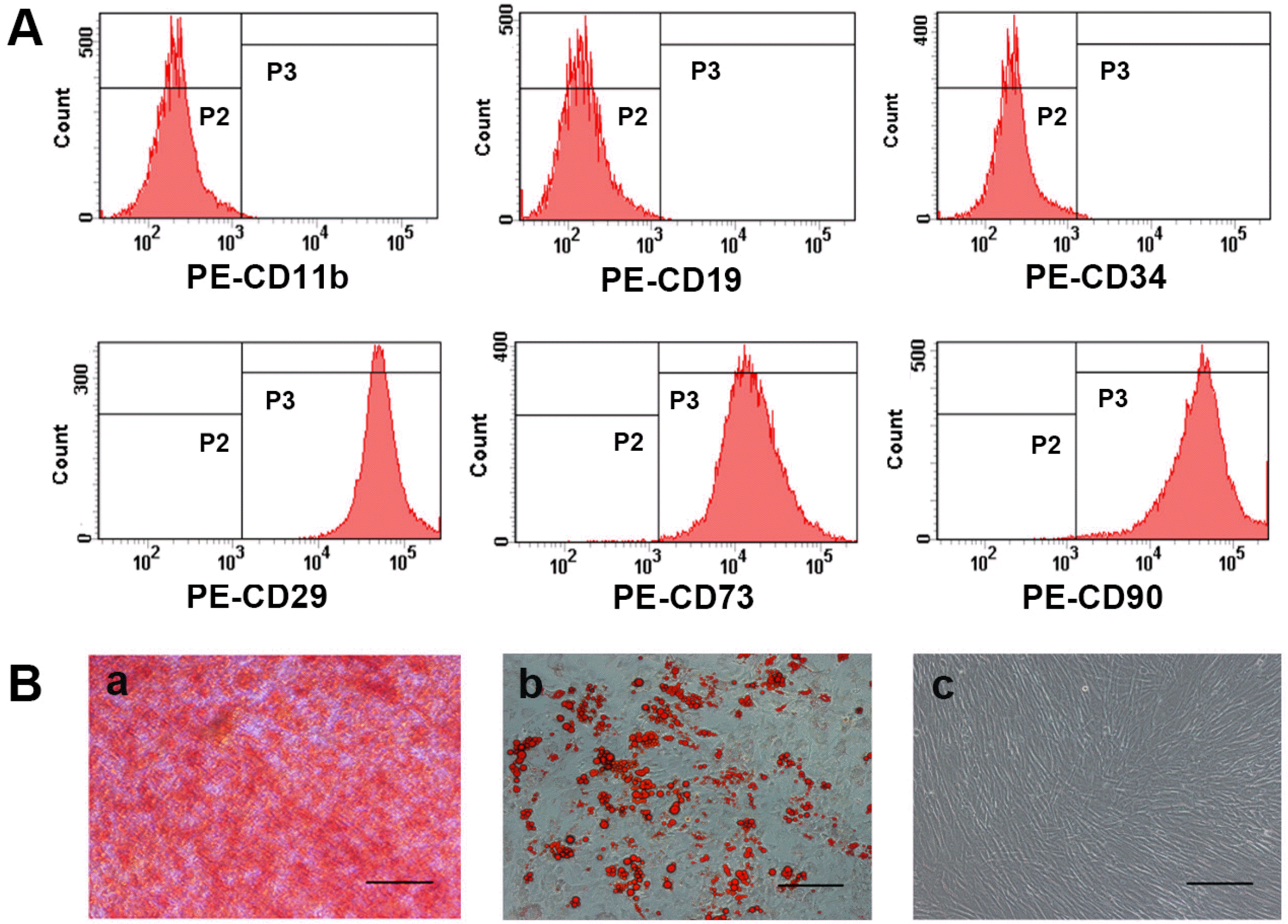 | Fig. 1Characterization of hUC-MSCs. (A) Flow cytometry analysis of surface antigens of hUC-MSCs. hUC-MSCs were negative for CD11b, CD19 and CD34, but positive for CD29, CD73 and CD90. (B) Differentiation potential of hUC-MSCs. (a) Osteogenic differentiation results using Alizarin Red staining, numerous cells became Alizarin Red positive. (b) Adipogenic differentiation results using Oil Red O staining, some of the cells contained numerous Oil Red O-positive lipid droplets. (c) Non-sti-mulated control cells grown in regular medium. (a∼c) Magnification, ×100; scale bar, 200 μm. |
hUC-MSCs repaired lung injury in SU5416-induced emphysema
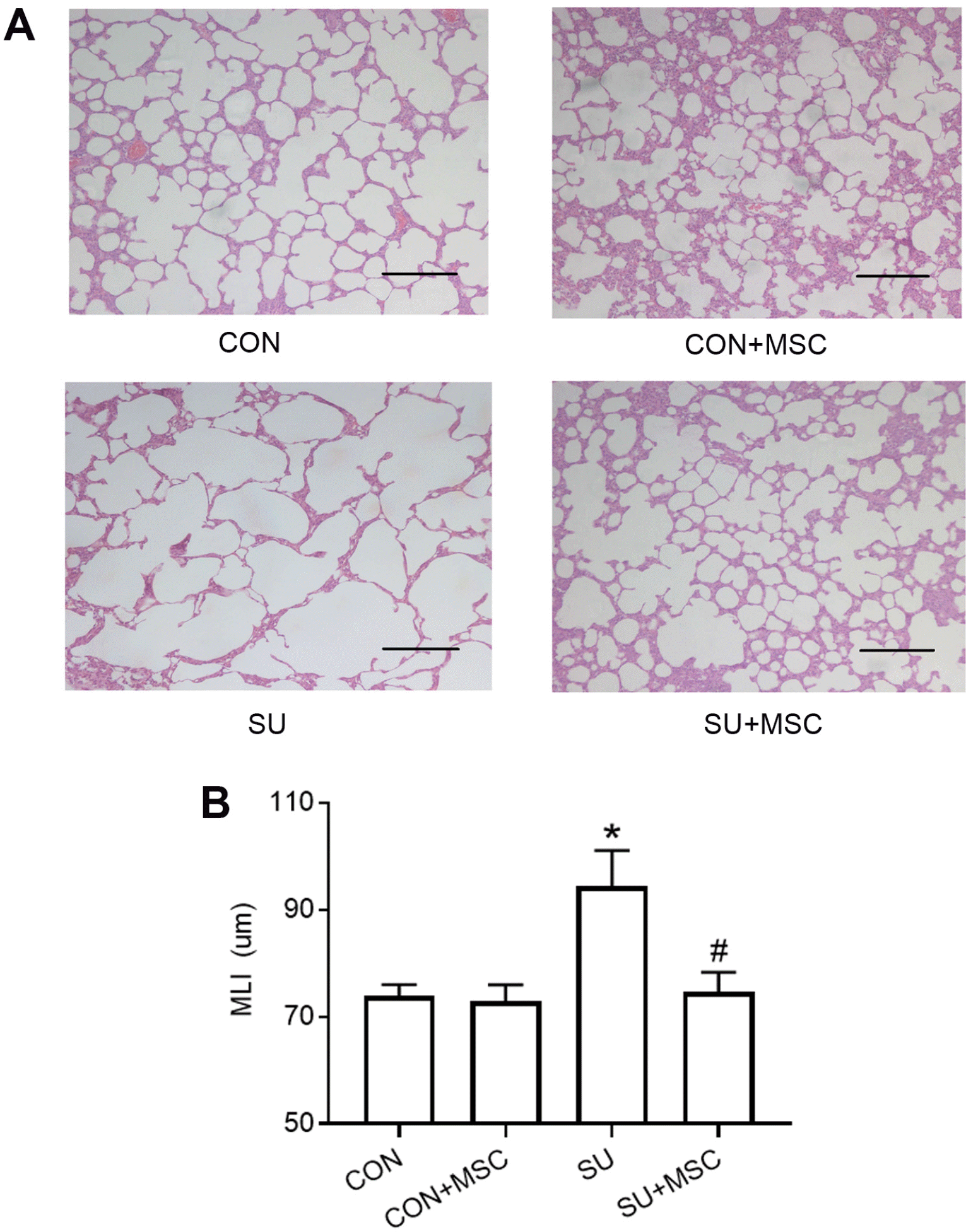 | Fig. 2hUC-MSCs repaired SU5416-induced emphysema. (A) Representative results of HE staining in lung sections chosen from each group. There was no significant difference between the Control group (CON) and the Control+MSC group (CON+MSC). Emphysematous changes were observed in the group treated with SU5416 (SU). hUC-MSCs transplantation repaired SU5416-induced emphysema (SU+MSC). Magnification, ×100; scale bar, 200 μm. (B) Morphometric analysis of the mean linear intercept (MLI). Values of MLI are presented as mean±SEM. *p<0.05 compared with the Control group. #p<0.05 compared with the SU5416 (SU) group. n=9. |
hUC-MSCs inhibited apoptosis in SU5416-induced emphysema
 | Fig. 3hUC-MSCs inhibited apoptosis of SU5416-treated lungs. (A) Re-presentative results of TUNEL assay using lung sections chosen from each group. There was no significant difference between the Control (CON) group and the Control+MSC (CON+MSC) group. Increased TUNEL-positive cells were observed in the group treated with SU5416 (SU). hUC-MSCs reduced TUNEL-positive cells (SU+MSC). Magnification, ×400; scale bar, 50 μm. (B) Quantitative results of TUNEL assay showing apoptosis index of the four groups. Data are presented as mean±SEM. ****p<0.0001 compared with the Control (CON) group, ####p<0.0001 compared with the SU5416 (SU) group. n=3. (C) Representative results of active caspase-3 and PCNA as determined by western blotting. hUC-MSCs inhibited increased levels of active caspase-3 but not affect proliferation in the SU5416-treated models. |
hUC-MSCs rescued VEGF expression in SU5416-induced emphysema
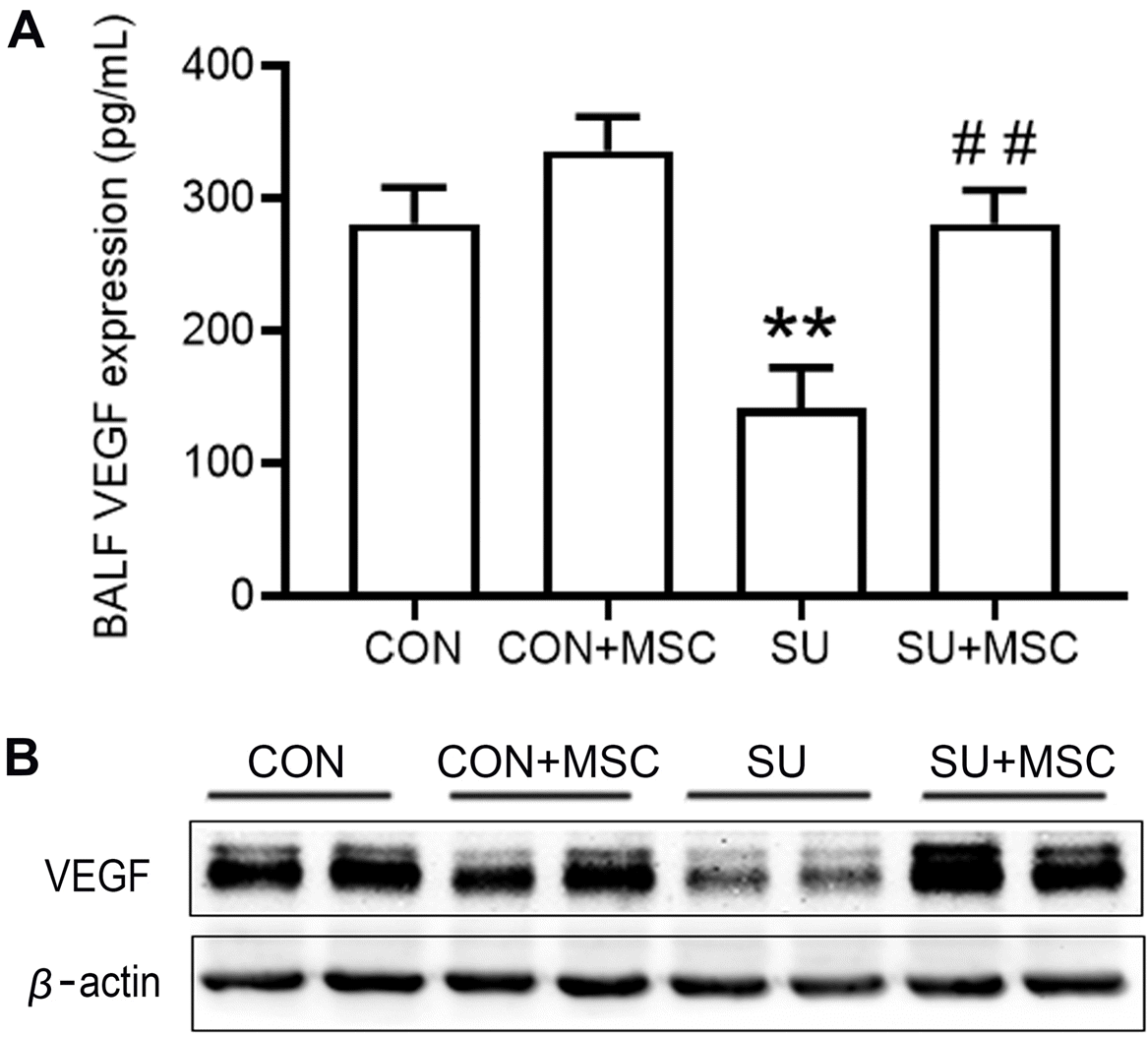 | Fig. 4hUC-MSCs rescued VEGF expression in SU5416-induced emphysema. (A) VEGF protein level in BALF was determined by ELISA. Data are presented as mean±SEM. **p<0.01 compared with the Control (CON) group, ##p<0.01 compared with the SU5416 (SU) group. n=9. (B) VEGF protein level in lungs as determined by western blotting. |
hUC-MSCs activated VEGFR2 and AKT in SU5416-induced emphysema
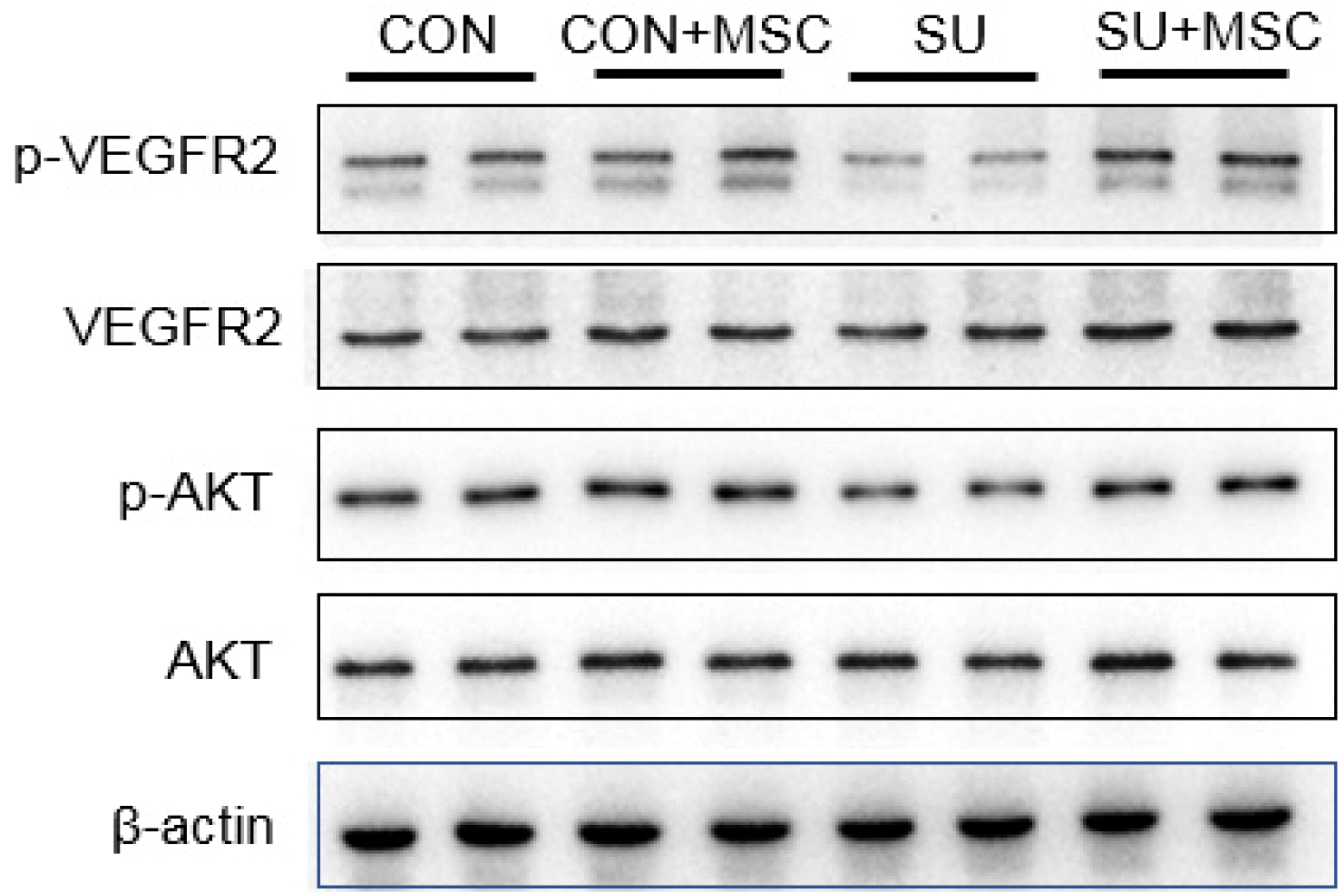 | Fig. 5hUC-MSCs rescued the expression of p-VEGFR2 and p-AKT in emphysema induced by SU5416. The protein expressions of p-VEGFR2, VEGFR2, p-AKT and AKT in the lungs were determined by western blotting. Compared with the control group (CON), p-VEGFR2 and p-AKT in the model group (SU) were significantly reduced, but both of them in the hUC-MSCs transplantation group (SU+MSC) returned to the level of the Control group. There was no significant change in the expression of VEGFR2 and AKT in each group. |
Expression of pro-inflammatory cytokines in lungs of experimental rats
 | Fig. 6hUC-MSCs did not alter the expression of pro-inflammatory cytokines in SU5416-induced emphysema. (A∼C) RQ-PCR detect IL-1β, IL-6 and TNFα mRNA expression in lungs, respectively. (D∼F) ELISA detect IL-1β, IL-6 and TNFα protein expression in BALF, respectively. hUC-MSCs had no significant effect on inflammatory response in normal or damaged lung tissues. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download