Introduction
Irreversible bladder damage often occurs in patients who experience congenital anomalies, chronic inflamma-tion, neuropathic disorder, or cancer invasion (
1,
2). Considering the particularity of its structure and physiological characteristics, an injured bladder cannot regain its initial morphology, and reconstructive surgeries of the bladder are often necessary for patients with these problems (
3). Therefore, the search for suitable substitutes for bladder tissue has recently intensified. In urethral tissue engineering, an ideal substitute for bladder tissue would be one lined with specialized epithelial cells known as urothelium (
4). Urothelium is present on the surface of the renal pelvis, ureters, bladder, upper urethra, and glandular ducts of the prostate. It functions not only as a protective barrier but also participates in chemical signaling (
5,
6). In treating these diseases, urological tissue engineering proposes combining urothelial cells with a biological scaffold material
in vitro culture and transplanting the combined material into the injured urethral area, ultimately leading to biological repair (
7-
10).
However, determining the appropriate urothelium source has been controversial. Recently, regenerative therapy by stem cell transplantation has been gradually adopted in the restoration of damaged tissues and organs in a variety of diseases (
11). In 2006, Takahashi and Yamanaka were the first to use transcription factors to reprogram adult-derived fibroblasts into induced pluripotent stem cells (iPSCs), which are similar to embryonic stem cells (
12). The iPSCs are well known for their powerful multiple differentiation ability (
13). Previous reports have indicated that iPSCs possess the potential to differentiate into the 3 germ layers and contribute to ophthalmology, neurology, and other tissue engineering clinical practice areas (
14). However, in the field of urethral injury repair, research regarding the cultivation of urothelial cells from iPSCs is lacking.
In this study, we summarized our own experiences and provided a method of differentiating mouse-iPSCs (miPSCs) into urothelial cells and explored this unique technology in its capacity to contribute to the biological repair of urethral injury.
Materials and Methods
The miPSCs were used in the differentiated animal models due to their stability
in vitro and the fact that they do not present the ethical challenges that arise with human iPSCs. According to previous reports, urothelial cells are differentiated from the posterior definitive endoderm (DE) (
15). In this study, the miPSCs were first induced to differentiate into posterior DE via addition of glycogen synthase kinase-3β (GSK3β) inhibitor, which was subsequently induced to differentiate into urothelial cells. The miPSCs were obtained from the China Agricultural University School of Biology (Beijing, China). And the mouse urothelial cells and mouse colon in our manuscript was purchased from iCell Bioscience (MIC-iCell-u007 and MIC-iCell-d007).
Cell culture
Gelatin (STEMCELL Technologies, China) and murine embryo fibroblast (MEF) were placed into 24-well plates 1 day before the experiment’s initiation. One day later, the miPSCs were grown at a density of 1×105 cells/well on a feeder layer of irradiated MEFs. The medium consisted of 80% high glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 15% fetal bovine serum (FBS; Gibco), 100 U/ml antibiotics (penicillin/streptomycin; Gibco), 0.1 mM β-mer-captoethanol (Gibco), 1,000 U/ml mouse leukemia inhibitory factor (LIF; MilliporeSigma, Burlington, MA, USA), 1 mM sodium pyruvate (Gibco), 0.1 mM MEM Non-Essential Amino Acids (MEN NEAA; Gibco), 2 mM Gluta/Max (Gibco), 3 uM CHIR99021 (GSK3β inhibitor, Tocris Bioscience, Bristol, UK), and 1 uM PD153035 (PD; MedChemExpress, Monmouth Junction, NJ, USA). The cells were maintained at 37℃ and 5% CO2, and changed once daily. When the cell fusion rate reached 80%, cells were passaged at a density of 4×105 cells per 3.5-cm dishes. The cells were routinely trypsinized and passaged at a ratio of 1:6 every 3 days. The Ethics Committee of the Affiliated Yantai Yuhuangding Hospital of Qingdao University (Yantai, China) approved this study.
Differentiation into posterior DE
According to previous research (
15), a procedure of differentiation into endoderm was employed. The miPSCs were digested by 0.5× TrypLE Select and then transferred from MEF to 24-well plates (2×10
5cells/well) coated with Gelatin (STEMCELL Technologies, China) 2 days prior to experiments. The cells were incubated at 37℃ and 5% CO
2 for 1 day. After 24 hours, the medium was replaced with Roswell Park Memorial Institute-1640 (RPMI-1640) medium containing 15% FBS, 100 ng/ml of Activin A (human, recombinant), and 6 uM CHIR99021. The cells were then cultured at 37℃ and 5% CO
2 for 3 days, with the medium being changed every day.
Differentiation into urothelial cells
The posterior DE medium was switched to RPMI-1640, in which there was 10 μM of all-trans retinoic acid (ATRA; Beinuo Biology, China) and 100 ng/ml of recombinant mouse fibroblast growth factor-10 (FGF-10; MedChemExpress). The medium was changed every 2 days. On day 12, the medium was supplemented with 1 μM troglitazone (TZ; MedChemExpress) and 1 μM PD153035 (PD; MedChemExpress) to induce terminal differentiation, and was changed every day until day 16.
Quantitative real-time polymerase chain reaction
According to the manufacturer’s instructions, RNA was isolated using Trizol reagent (Tiangen Biotech, China). RNA was then reverse transcribed by PrimeScript RT enzyme mix and RT Primer Mix (Accurate Biology, China). Quantitative real-time Polymerase Chain Reaction (qRT-PCR) was applied to quantitative analysis using TB Green Premix Ex Taq II, PCR Primer, and ROX Reference Dye (QIAGEN, Hilden, Germany)on an Automated Thermal Cycler instrument (Thermo Fisher Scientific, Waltham, MA, USA). Mouse GAPDH served as the internal control. All qRT-PCR data were analyzed using the ΔΔCt method. The qRT-PCR primers are presented in
Table 1. The qRT-PCR experiments were repeated using six biological replicates.
Table 1
|
Gene name |
Forward |
Reverse |
|
GAPDH |
CATCACTGCCACCCAGAAGACTG |
ATGCCAGTGAGCTTCCCGTTCAG |
|
OCT4 |
CAGCAGATCACTCACATCGCCA |
GCCTCATACTCTTCTCGTTGGG |
|
NANOG |
GAACGCCTCATCAATGCCTGCA |
GAATCAGGGCTGCCTTGAAGAG |
|
SOX2 |
AACGGCAGCTACAGCATGATGC |
CGAGCTGGTCATGGAGTTGTAC |
|
CDX2 |
CATCAGGAGGAAAAGTGAGCTGG |
TTTTCCTCTCCTTGGCTCTGCG |
|
SOX17 |
GCCGATGAACGCCTTTATGGTG |
TCTCTGCCAAGGTCAACGCCTT |
|
FOXA2 |
CGAGCACCATTACGCCTTCAAC |
AGTGCATGACCTGTTCGTAGGC |
|
HOXA13 |
CCCAAAGAGCAGACGCAGCCT |
GTGTAAGGCACGCGCTTCTTTC |
|
HOXD13 |
ATCAGCCACAGGGGTCCCATTT |
GAGCTGCAGTTTGGTGTAAGGC |
|
Uroplakin IA |
GGATTGCCATCTTCTGCGGCTT |
TGATGCAGGAGGCACACTCGAA |
|
Uroplakin IB |
CCTGAAGCAGATGCTGATGAGG |
TGCCAGTCTGACGGACCGTTTA |
|
Uroplakin II |
AGAGACTCCCATGTCCACGCTT |
CAGCACTGTGATGACCACCATC |
|
UPK III |
GTTTCGCTGGAGCCATCATCCT |
CGGTTCACAGATGAGTAGGAAGG |
|
Uroplakin IIIB |
GTGCTTCGGGTGGGCAATGATT |
TGGACCACTTCGTCTCAGCCTT |
|
Cytokeratin 20 |
GGATTCGAGGTTCAAGTCACGG |
TCTAGGTTGCGCTCCAGAGACT |
|
Cytokeratin 5 |
GAACAGAGGCTGAGTCCTGGTA |
TCTCAGCCTCTGGATCATTCGG |
|
Cytokeratin 7 |
CGGAGATGAACCGCTCTATCCA |
CATGAGCATCCTTGATTGCCAGC |
|
Cytokeratin 13 |
GACTGGCATCTGAAACAGAGCC |
TTGTCCGTGGTGGCTTCCAGAA |
|
ZO-1 |
GTTGGTACGGTGCCCTGAAAGA |
GCTGACAGGTAGGACAGACGAT |
|
E-cadherin |
GGTCATCAGTGTGCTCACCTCT |
GCTGTTGTGCTCAAGCCTTCAC |

Flow cytometry
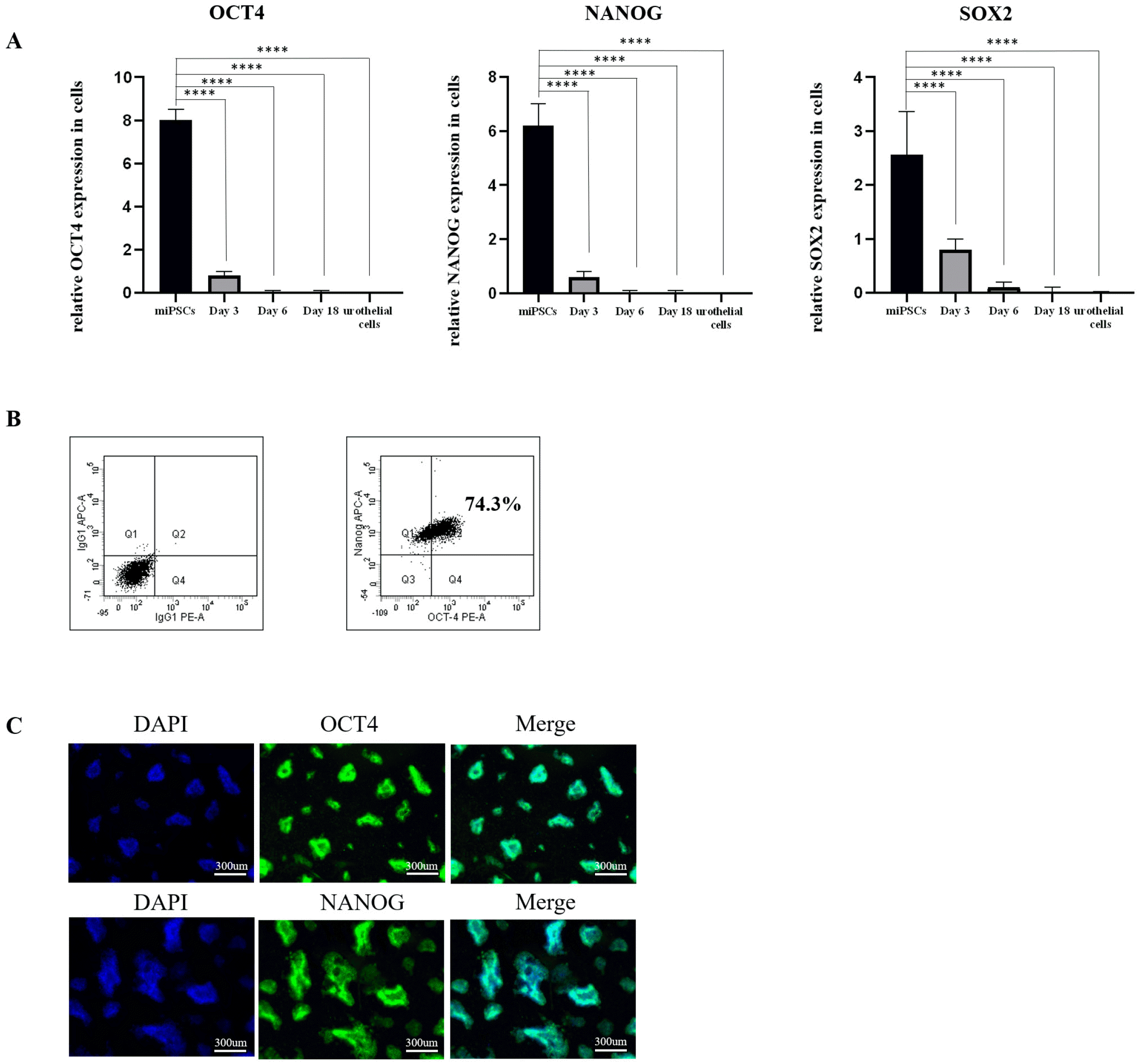
Cells were cultured in a 6-cm dish and trypsinized in a single-cell suspension. The 105 cells were added to sample tubes, which was followed by the addition of 100 ul of membrane-breaking fixative solution A and fixation for 15 minutes at room temperature in the dark. After 3 washes with phosphate-buffered saline (PBS), cells were centrifuged at 1000 rpm for 5 minutes, and the supernatant was discarded. The cells were labeled with fluorophore-conjugated antibodies on ice for 30 minutes. Anti-bodies were used according to the manufacturer’s instruc-tions. Then, membrane-breaking fixative solution B was added, and followed by incubation for 15 minutes in the dark at room temperature. After another centrifugation at 1000 rpm for 5 minutes, the supernatant was discarded. After a wash with PBS, the flow cytometry results were analyzed with BD FACS Canto II (BD Biosciences, Franklin Lakes, NJ, USA) The reagents used in the experiment were PE anti-Oct4 (Oct3) Antibody (dilution 1:100; BioLegend, San Diego, CA USA), Alexa Fluor 647 anti-Nanog Antibody (dilution 1:100; BioLegend), PE Mouse IgG2b, κ Isotype Ctrl Antibody (BioLegend), and Alexa Fluor 647 Mouse IgG1, κ Isotype Ctrl (ICFC) Antibody (BioLegend). The Flow cytometry experiments in each group were repeated at least three times.
Immunofluorescence staining
Immunofluorescence staining was performed to analyze the expression of pluripotency markers (OCT4, NANOG), posterior DE markers (CDX2, SOX17, FOXA2), caudal hindgut markers (HOXD13), and urothelial markers (uropla-kin (UPK) II, CK7, CK13, CK20, ZO-1 and E-Cadherin) (The antibodies were presented in
Supplementary Table S1). The cells were fixed in 4% paraformaldehyde at room temperature and washed 3 times with PBS. After 15 minutes, the cells were permeabilized with 0.2% Triton X-100 (Solarbio Life Sciences, China) for 20 minutes and washed 3 times with PBS. After blocking with 5% bovine serum albumin (BSA; Biotopped Life sciences, China), a primary antibody was added to each well. The cells were then were incubated overnight at 4℃ in a wet box. Subsequently, after being rinsed with PBS, the cells were incubated with goat anti-rabbit secondary antibody for 60 minutes. DAPI (4’,6-diamidino-2-phenylindole) staining was used to investigate the cell nuclei. Fluorescent labeling was observed under a fluorescent microscope (ECHO, USA). The primary antibodies are presented in
Supplementary Table S1. The Immunofluorescence staining experiments in each group were repeated at least four times.
Western blotting
Western blotting was performed using the standard methods. Total protein was extracted using Radio Immu-noprecipitation Assay (RIPA) Lysis buffer (Servicebio Technology, China) with phenylmethanesulfonyl fluoride (PMSF; Sparkjade Scientific, China). An equal amount of protein was separated on 10% Bis-Tris gels (Thermo Fisher Scientific) and transferred onto a nitrocellulose membrane by electroblotting. The membrane was incubated overnight at 4℃ in primary antibody, rinsed 3 times with tris-buffered saline with Tween 20 (TBST), and incubated with a second antibody for 1 hour at room temperature the next day. The membranes were then subjected to a developer for imaging using a luminous solution (Affinity Biosciences, Cincinnati, OH, USA). The primary antibodies are presented in
Supplementary Table S1. The Western blot experiments in each group were repeated at least five times.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, UA), and a p value <0.05 was considered statistically significant (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001). n indicates technical repeats, which were verified by three or six independent experiments.
Discussion
At present, numerous urological diseases ultimately require surgical reconstruction as treatment. The preferred replacement protocol calls for the use of intestinal tissue. Since the functions of gastrointestinal epithelium and urothelium are different in nature, the incidence of postoperative complications is accordingly high (
5). Therefore, the selection of appropriate seed cells is critical for the repair of urinary system lesions. Oottamasathien et al. (
20) reported that mouse embryonic stem cells possess the ability to differentiate into urothelial cells. Thus, we hypothesized that iPSCs can be used as seed cells to reconstruct the urinary system.
Compared with traditional stem cells, iPSCs exhibit features of easy derivation and multipotent differentiation (
12). Kang et al. (
21) stated that it is possible to generate living mice entirely from iPSCs through tetraploid blastocyst complementation, which is considered to be the most stringent assay for evaluating the pluripotency of stem cells. Multiple cell types can be successfully dedifferentiated into iPSCs, which are used in various research fields (
7,
22). Bar-Nur et al. (
23) reported that human-induced pluripotent stem cells (hiPSCs) could differentiate into insulin-producing cells. Several studies have also reported that iPSCs can be used for experimental models of Parkinson’s disease (
11,
24). Therefore, the induction of iPSCs into urothelium would suggest new options for urinary tissue engineering.
The term
urothelium was first introduced by Melicow in 1945, and urothelium is 1 of the 8 known epithelial tissue types
in vivo. From the basement membrane to the lumen, the urothelium is a stratified epithelium composed of 3 cell types: the superficial layer composed of a single layer of umbrella cells, the intermediate cell layer composed of single or multiple layers depending on species, and the basal cell layer (
25). Umbrella cells are highly differentiated cells and works as a barrier and as means of information transmission (
26). The tight junctions, apical glycan layer, and urothelial cell-specific uroplakin proteins cooperatively maintain the barrier function of the umbrella cell layer (
27). Intermediate cells are connected to the adjacent surface and basal cell layers through desmosomes. The intermediate cell layer consists of partially differentiated cells that cells are responsible for repairing urothelium damage (
27). The basal cell layer is composed entirely of mononucleates, to which it self-renewal potential is attributable.
The primary embryonic disc of vertebrates are disc-shaped structures that contain cells of the 2 germ layers: epiblasts and hypoblasts. After proliferation and migration, some of the epiblasts gradually form the anterior primitive streak (APS), which then gradually differentiated into the DE. The formation of the bladder and urethra starts when the DE is encapsulated to form the primitive gut. The hindgut is the tail end structure of the primitive gut, and its terminally expanded portion is called the cloaca (
28). The ventral and dorsal sides of the cloaca form the urogenital sinus and the anal canal, respectively. The bladder and urethra are generated from differentiation of the urogenital sinus (
29).
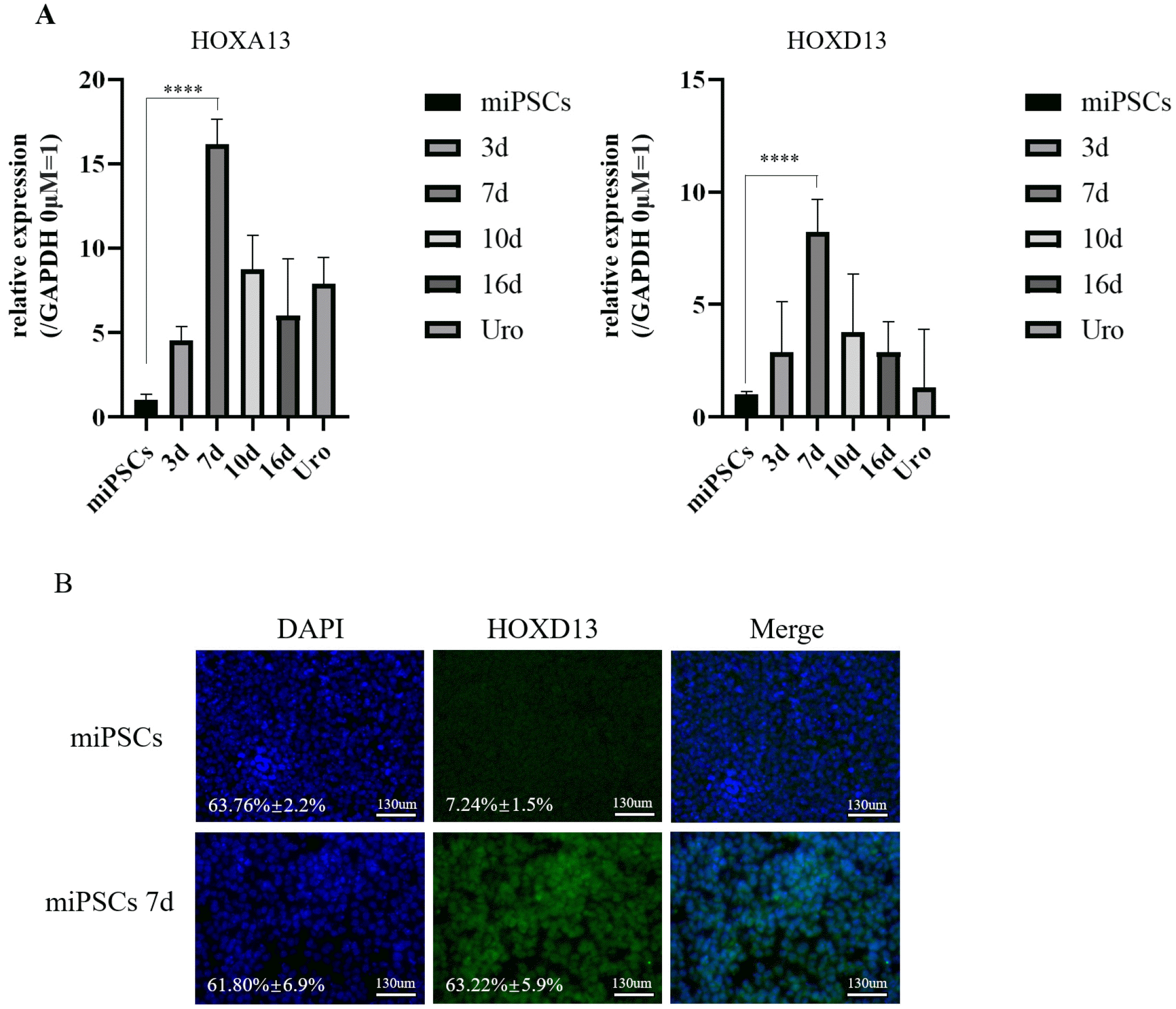
Although the specific differentiation process is unclear, there are 3 important phases in the process of differentiation: posterior DE, caudal hindgut, and urothelial cells (
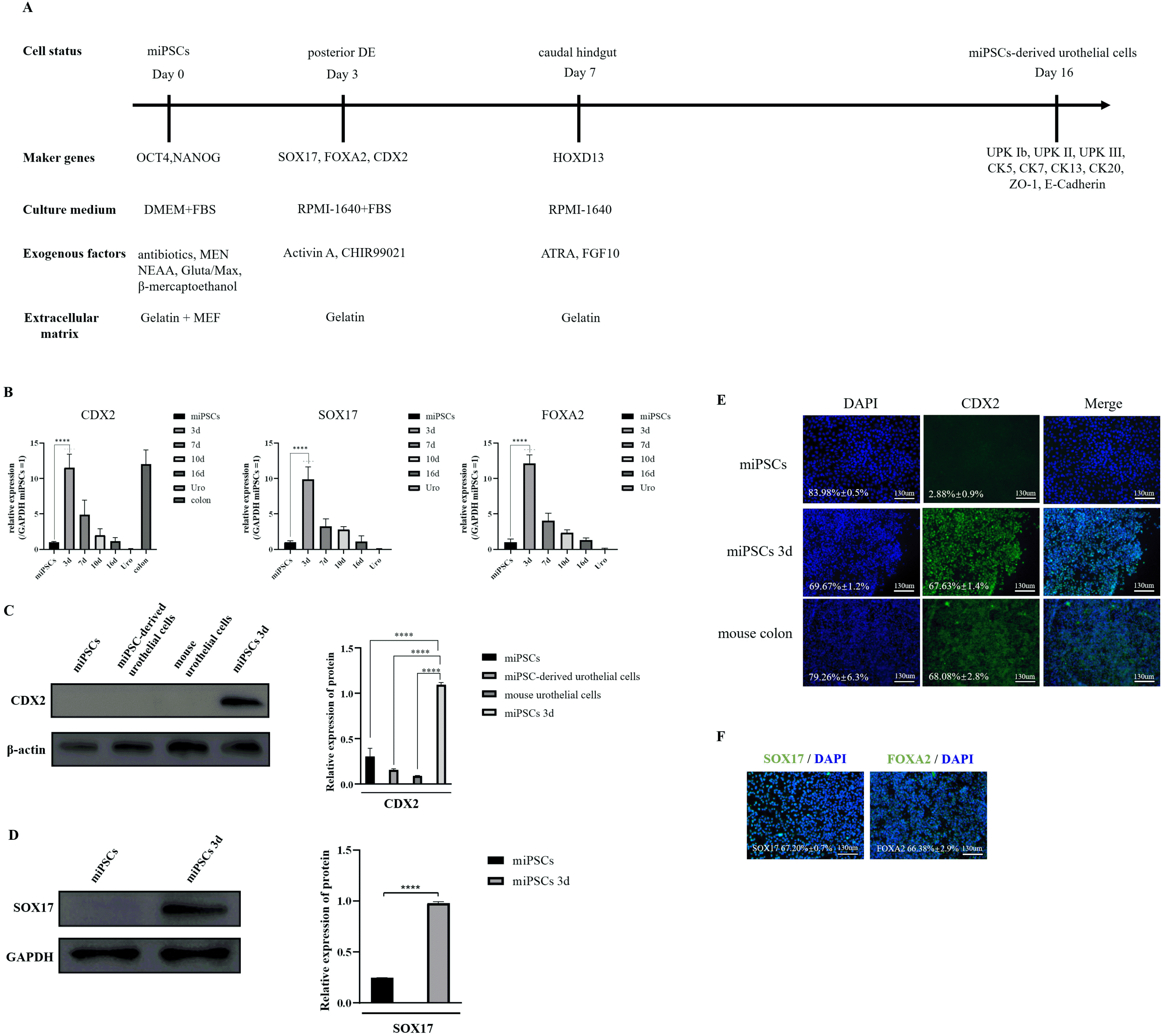
16). The CDX2 gene is the caudal family’s homeobox gene and is the marker gene for posterior DE (
30). Transcription factor SOX17 is the DE marker gene, and its downstream target FOXA2 has also been considered to be the key DE marker. UPKs are specialized transmem-brane proteins that are considered to be markers of mature urothelial cells (
31,
32). There are 4 subtypes of UPKs (UPK IA, UPK IB, UPK II, and UPK III), which exist in a heterodimer form (
27). Deng et al. (
33) observed that urothelial-associated glycoprotein (P35) is similar to UPK III in terms of its morphological characterization and biological functions. They also classified UPK III into 2 subtypes (UPK IIIA and UPK IIIB). Its expression was closely related to the function of urinary tract epithelium. The loss of UPK III could lead to the defect of barrier function. It had been reported that the deletion of UPK IIIa leaded to a significant increase in bladder capacity, micturition pressure and demonstrable nonvoiding contractions in mice, and interacted with UPK II to affect the differentiation of urothelial cells (
34). Both UPK IIIb and UPK IIIa could be co-expressed in urothelial cells and were specifically expressed both in development as well as under homeostatic conditions (
35). UPK IIIb deficiency in mice could affect the development and integrity of bladder and upper genitourinary system (
33). And its binding to UPK Ib was the key to the early steps of urothelial plaque assembly. The barrier function of urothelial cells is guaranteed by the presence of UPKs (
31,
34).
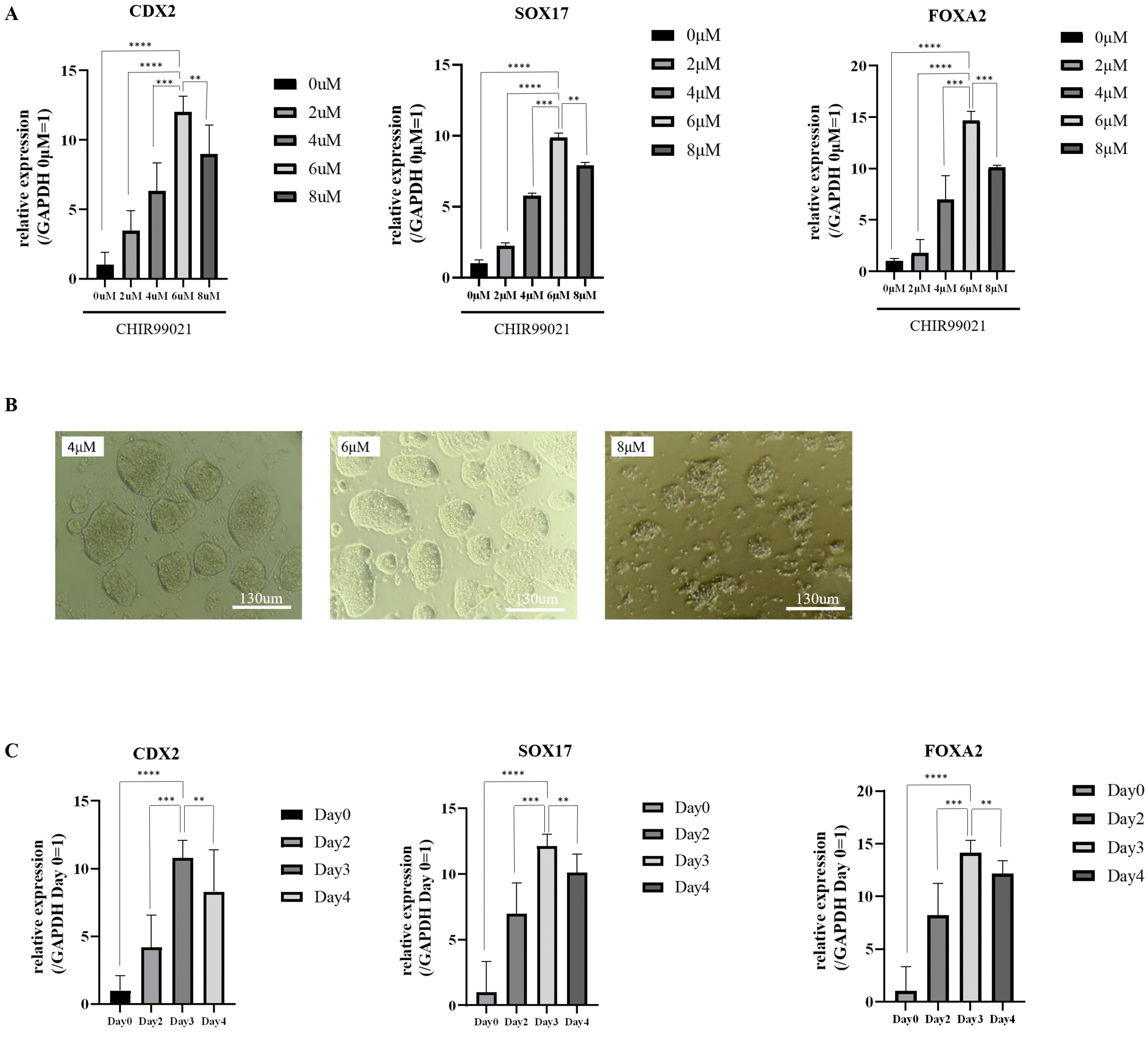
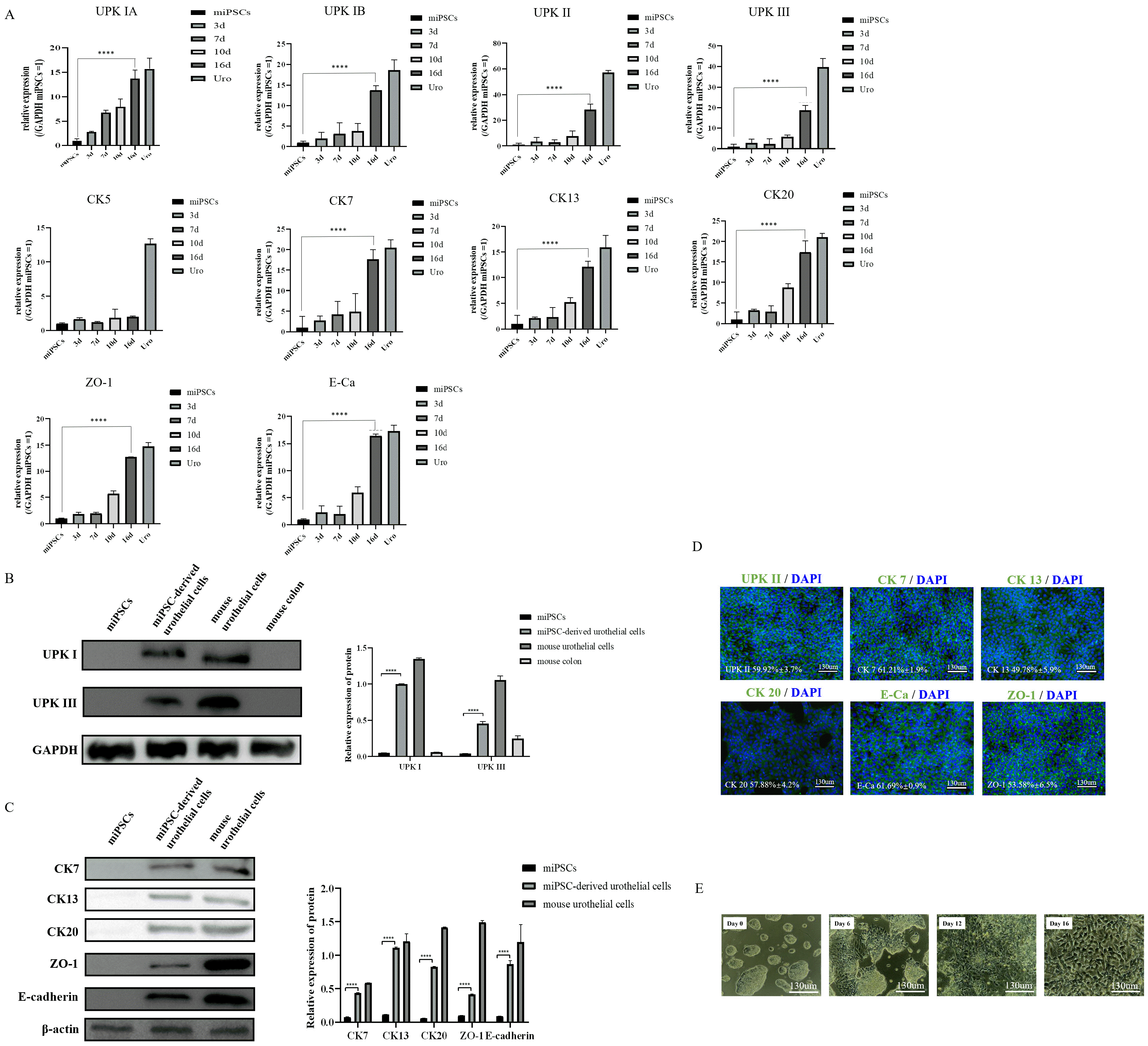
To the best of our knowledge, no reports regarding the differentiation of mouse-derived iPSCs into terminally differentiated monolayer of apical urothelial cells exist. In the present study, we established an optimal method for cultivating miPSCs in vitro and succeeded in differentiating miPSCs into urothelial cells using 2 different mediums with inducers. We induced the differentiation of miPSCs into posterior DE using a suitable concentration of the GSK3β inhibitor, CHIR99021. After 3 days of differentiation, the cells expressed three posterior DE biomarkers (CDX2, SOX17, and FOXA2). The high expression of both caudal hindgut markers (HOXA13 and HOXD13) was observed in cells after 7 days of differentiation. The mature urothelial cell markers [umbrella urothelium markers UPK Ib, II, III, CK20, CK7, and CK13), and tight junction molecules (ZO-1 and E-Cadherin)] were highly expressed in differentiation-induced miPSCs on day 16, indicating that the miPSCs had differentiated into urothelial cells. To efficiently and robustly differentiate miPSCs into urothelial cells, we determined the optimal concentration and suitable duration of CHIR99021 use in miPSCs.
Wnt/β-catenin signaling was strongly associated with induction of differentiation of the original gut tube into distinct gut tube regions and extension of the hindgut. Similar results have been reported in
Xenopus laevis: the inhibition of Wnt/β-catenin in the anterior endoderm maintains the formation of the foregut, while the highly active Wnt/β-catenin in the posterior endoderm inhibits the generation of the foregut and enhances the development of the intestine (
36). CHIR99021 activates Wnt signaling pathway via suppressing GSK3β and preventing the formation of β-catenin destruction complex (
18). It was also reported that appropriate concentration of CHIR99021 could induce the differentiation of human pluripotent stem cells into posterior DE (
17). The vitamin A–mediated signaling pathway plays a key role in the formation of the bladder from the urogenital sinus as well as in the maintenance of the differentiated urothelial phenotype. Batourina et al. (
37) found that mice with disorders of embryonic retinoic acid show a highly abnormal rate of urogenital sinus. ATRA is the main bioactive derivative of vitamin A and participates in the differentiation of endoderm during development (
38).
The generation of urothelial tissue from human-derived pluripotent stem cells has been previously reported (
15). Compared with hiPSCs, miPSCs are easily obtainable and more conducive to follow-up experiments
in vivo. It is hard to perform relevant
in vivo experiments using human-iPSCs due to the influences of many factors, including patients willingness, ethical approval, and the operationalization of subsequent validation experiments. For this reason, developing a well-established animal model to further explore the application of iPSCs for urethral tissue engineering is crucial and may provide a basis for further research into human-iPSCs. Human and mouse genomes share similar long-range sequence organization, with most of their genes being homologous. Mouse animal models have attracted increasing attention in tissue engineering. However, the low survival rate and low differentiation efficiency of miPSCs hinder its progress. The miPSCs in our model were of murine origin, and our protocol is stable and reproducible. Thus, for the first time, a feasible protocol to obtain urothelium from miPSCs is described in our study, which may provide reference for differentiation of mouse-iPSCs to urothelial cells for future research.
However, our study had some limitations that should be noted. First, there was a lack of further experiments to verify the biological functions of miPSC-derived urothelial cells. Thus, the clinical applications of this protocol may be constrained due to the miPSC-derived urothelial cells from our protocol being a monolayer of apical urothelium. Furthermore, the mechanism of miPSC differentiation into urothelial cells remains to be elucidated. In future studies, we will conduct further experiments to explore and address the issues above.
In conclusions, This study successfully induced miPSC differentiation into terminally differentiated monolayer of apical urothelial cells in vitro and optimized the induction system. Therefore, these results may provide a theoretical basis for the application of miPSCs in vivo tissue engineering experiment.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download