Introduction
The regeneration of periodontal tissue requires abundant capillaries to maintain the blood and nutrient supply. The newly formed capillaries provide oxygen, nutrients and progenitor cells for the damaged area, thereby promoting regeneration. Iwasaki et al. (2014) (
1) transplanted human periodontal ligament stem cells (PDLSCs) with acellular amniotic membranes into bifurcation defects caused by surgery, four weeks after transplantation, the defect in the control group was mainly filled with fibroblasts, collagen fibers and blood vessels, and new bone and dentin were formed, periodontal membrane stem cells have positive effects on vascular regeneration, bone regeneration and fiber regeneration in tissue defects. Perio-dontal tissue regeneration is regulated by various cytokines and pathways. In addition to the formation of new bone, the development of capillaries plays an important role in promoting osteogenesis. The biological regulation of PDLSCs during tissue regeneration may not be a one-way effect and may instead be the result of multiple interactions.
PDLSCs have multidirectional differentiation potential and are favored in the field of regenerative medicine as seed cells. In studies related to PDLSCs and endothelial cells, PDLSCs are often cocultured with human umbilical vein endothelial cells (HUVECs) to construct an
in vitro model. However, there are functional differences among endothelial cells derived from periodontal ligament tissue, gingival tissue and vascular endothelial cells (
2). The periodontal tissue environment is complex, and tissue surrounding the teeth changes during the progression of periodontitis, which is not limited to the repair of surrounding bone tissue defects. Endothelial cells derived from the periodontal ligament and the surrounding capillary network are similar to the actual periodontal tissue microenvironment for endothelial and related research. PDLSCs are present around the vascular plexus of the periodontal ligament, participate in the maintenance of vascular-like structures (
3), and have the potential for endothelial differentiation (
4). Differentiated endothelial-like cells can participate in angiogenesis. The endothelial differentiation of PDLSCs may affect the repair and regeneration of periodontal tissue, but whether angiogenesis after endothelial differentiation can assist in the repair and regeneration of periodontal tissue is critical.
The formation of blood vessels requires two endothelial cell types: H-type and L-type cells. H-type and L-type blood vessels show some differences;
CD31 and endomucin (Emcn) are highly expressed in endothelial cells in H-type blood vessels and poorly expressed in endothelial cells in L-type blood vessels. High expression of
CD31 and Emcn in H-type blood vessels is beneficial for the formation of bone tissue. The abundance of H-type blood vessels decreases with age and is closely related to age-related osteoporosis in elderly individuals (
5,
6). Increasing the abundance of H-type blood vessels was shown to promote fracture repair (
7). In this study,
CD31 was used as an endothelial cell marker to examine the biological activity of endothelial-like cells after PDLSC differentiation.
LIPUS is a treatment method that uses mechanical sti-mulation generated by acoustic radiation force to act on cells and exert therapeutic effects. LIPUS can regulate the behavioral abilities of various stem cells, such as the proliferation (
8), differentiation (
9), and apoptosis (
10) of bone marrow mesenchymal stem cells (BMSCs) and dental pulp stem cells (DPSCs). LIPUS can also promote the proliferation of vascular endothelial cells (
11) and angiogenesis (
12). LIPUS can inhibit angiogenesis in tumors, affect the tumor blood supply, and hinder tumor development (
13). In cardiovascular and cerebrovascular diseases, LIPUS can promote angiogenesis and subsequent neurogenesis to promote the recovery of ischemic stroke neurons (
14). In this study, cells were physically treated by LIPUS to examine whether the endothelial-like cells differentiated from PDLSCs had the potential to form H-type blood vessels in response to LIPUS and the effect of LIPUS on angiogenesis of these cells.
Piezo1 is a nonselective cation channel in the mechanically sensitive ion channel family and is mainly expressed in nonsensing tissues associated with contact fluid pressure and flow. Physical stimulation induces ATP release, activates P2 receptors to regulate the function of urothelial cells, endothelial cells, and red blood cells, and enhances the migration of MSCs through the
Piezo1 channel (
15). In addition, periodontal ligament cells (PDLCs) can be induced by tensile mechanical stimulation and then activate
Piezo1 to participate in the mechanical transduction of PDLCs and promote the osteogenic differentiation of periodontal ligament cells (
16). In summary, LIPUS exerts mechanical force on cells by low-frequency pulse acoustic stimulation and may also participate in signal transduction from PDLSCs to endothelial cells through
Piezo1.
LIPUS can promote osteogenic differentiation in PDLSCs in inflammatory or noninflammatory conditions (
17), but its effect on the endothelial differentiation of PDLSCs and its correlation with periodontal tissue regeneration have not been examined. In this study, we hypothesized that LIPUS could regulate the endothelial differentiation of PDLSCs and the formation of microvessels to promote the regeneration and repair of periodontal tissue. We then preliminarily explored the mechanism of LIPUS to provide a theoretical basis for the clinical application of LIPUS.
Go to :

Materials and Methods
Cell culture and identification
This research protocol was approved by the Ethics Committee of the Affiliated Stomatological Hospital of Chongqing Medical University [No: 2020 (LSNO 65)]. All the included patients signed informed consent forms.
To ensure the optimal state of PDLSCs and exclude other interfering factors, such as aging, we collected healthy orthodontic teeth from patients aged 11∼20 years for PDLSC extraction, and sex was not specified. PDLSCs were scraped from periodontal ligament tissue in the middle 1/3 of the root, subjected to enzymatic digestion (Biosharp, Beijing, China), and cultured. The cells were cultured in α-MEM (Biosharp, Beijing, China) containing 10% fetal bovine serum (FBS) (Gemini, California, USA) and 1% penicillin and streptomycin (Biosharp, Beijing, China). The experiments used PDLSCs passaged for 3∼4 generations.
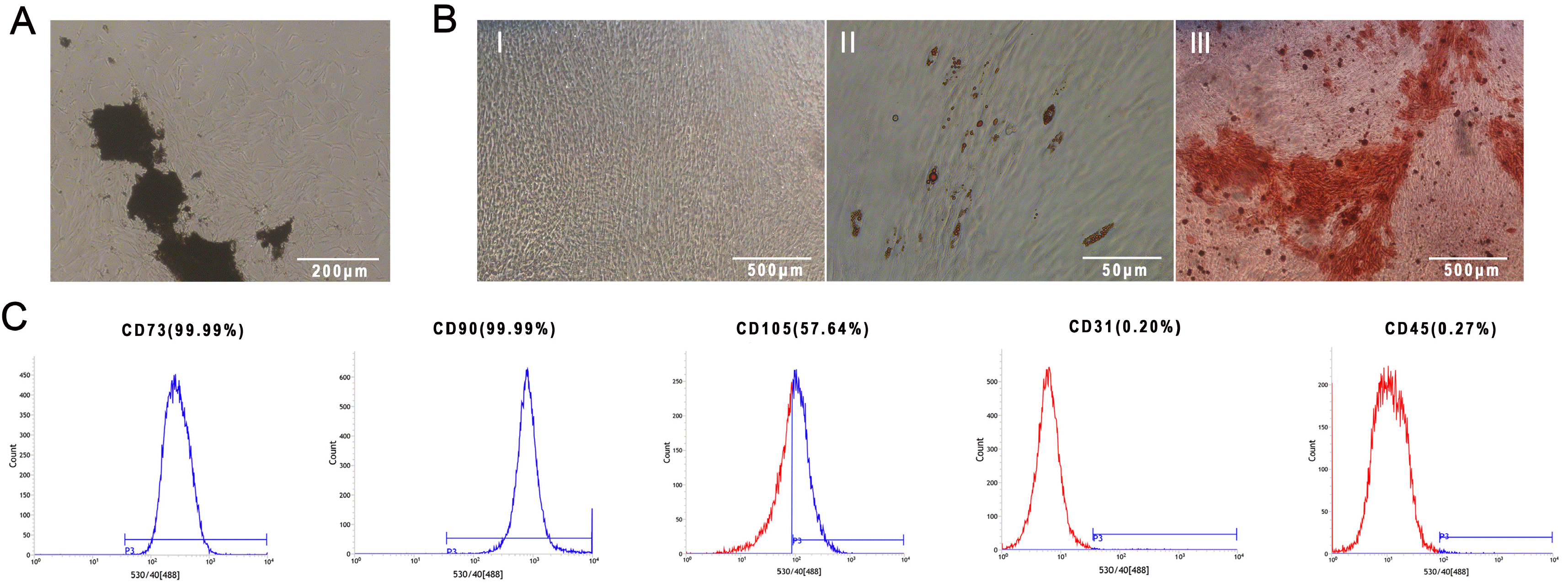
Flow cytometry was used to identify the expression of the antigens CD90, CD73, CD105, CD45, and CD31 on the cell surface, and their expression confirmed that the primary cultured PDLSCs had MSC characteristics.
Osteogenic induction and adipogenic induction were performed to confirm the multidirectional differentiation ability of PDLSCs. Osteogenic induction was performed by replacing the medium with α-MEM containing 10% FBS, L-ascorbic acid 2-phosphate (50 g/ml) (Solarbio, Beijing, China), dexamethasone (108 mM) (Solarbio, Beijing, China), and β-glycerophosphate (10 mM) (Sigma, USA). The formation of mineralized nodules was shown by alizarin red staining after osteogenic induction for 14 or 21 days. Adipogenic induction was performed using adipogenic induction medium with α-MEM containing 10% FBS, 100 μM indomethacin (Sigma, USA), 5 μg/ml insulin (Sigma, USA), 0.5 mM 3‐isobutyl‐1‐methylxanthine (Sigma, USA), and 1 μM dexamethasone (Sigma, USA), and lipids were stained with oil red O after adipogenic induction for 14 days.
Endothelial induction of PDLSCs was performed using endothelial induction medium with α-MEM containing 10% FBS, 25 ng/ml VEGF (Sino Biological, Beijing, China) and 25 ng/ml bFGF (Sino Biological, Beijing, China) for 14 days, and the induction medium was exchanged every 2∼3 days. The cells were divided into the groups named V (medium with VEGF and bFGF), LV [medium with LPS (Sigma, USA), VEGF and bFGF], VG [medium with GsMTx4 (MCE, USA), VEGF and bFGF], and LVG (medium with LPS, GsMTx4, VEGF and bFGF). All media were replaced every 2∼3 days.
LIPUS treatment
LIPUS equipment (Chongqing Haifu Medical Technology, Chongqing, China) was purchased from the Chongqing Ultrasonic Medical Engineering Research Center, China. The actual output power was tested and recommissioned with a UPM-DT-1 ultrasonic power meter. The output power of the ultrasound transducer was determined and corrected by Chongqing Ultrasonic Medical Engineering Research Center. The LIPUS parameters were as follows: the output frequency was 1.5 MHz, the intensity unit was mW/cm2, the pulse length was 200 μs, and the repetition rate was 1 kHz. The above parameters were based on our previous studies. The ultrasonic probe was fixed by a foam box to keep the ultrasound transducer probe surface standing and placed upward. Then, the medical ultrasonic coupling agent was evenly coated on the surface of the probe so that the probe surface directly contacted with the bottom of the dish, and the coupling agent fully contacted the probe surface. The irradiation time was 20 min/dish, and the dishes without LIPUS treatment were also exposed to the same working environment for 20 min to avoid the error caused by an inconsistent cell culture environment.
Flow cytometry
The cells were treated according to the corresponding experimental conditions and routinely digested, and the total cells were counted. The cells were washed 3 times, and each sample contained 1×106 cells in 100 μl aliquots. Cells were incubated with fluorescent antibodies including human CD31-FITC (1:100, Biolegend, USA), CD45-FITC (1:100, Biolegend, USA), CD73-FITC (1:100, Biolegend, USA), CD105-FITC (1:100, Biolegend, USA) and CD90-PE (1:100, Biolegend, USA) in the dark at 4℃ for 30 min. Untreated cells were used as a control group. Flow cytometry was performed on a BD AccuriTM C6 flow cytometer (BD Biosciences, USA). Positive expression was indicated by red. Cell differentiation rate=the number of fluorescent cells/the total number of detected cells×100%.
Quantitative real-time PCR
The gene expression of
GAPDH (TaKaRa, Japan),
ALP (TaKaRa, Japan),
CD31 (TaKaRa, Japan),
IL-6 (TaKaRa, Japan) and
Piezo1 (TaKaRa, Japan) were determined by quantitative reverse transcription polymerase chain reaction (RT-PCR). Cells prepared with the corresponding experimental conditions were digested thoroughly, and the total cells were counted. Approximately 5×10
5 cells were collected per sample. Total RNA was extracted from cells in different dishes with TRIzol
Ⓡ (TaKaRa, Tokyo, Japan), and miRNA was reverse transcribed to cDNA using PrimeScript
TM RT Master Mix (TaKaRa, Tokyo, Japan). RT-PCR was performed with SYBR
Ⓡ Premix Ex Taq II (Tli RNase H Plus; TaKaRa), and each sample was added to 3 wells. The primer sequences are shown in
Table 1-Primers used in this study.
GAPDH primers were used for normalization. Data were analyzed using the 2
−(ΔCt) method.
Table 1
Primers used in this study
|
Target gene |
Forward (top) and reverse (bottom) primer sequences |
|
CD31
|
5’-CCAAGATAGCCTCAAAGTCGG-3’ |
|
5’-CTGGGCATCATAAGAAATCCTG-3’ |
|
ALP
|
5’-CTTGACCTCCTCGGAAGACAC-3’ |
|
5’-CAGACCAAAGATAGAGTTGCCAC-3’ |
|
IL-6
|
5’-GAGTAGTGAGGAACAAGCCAGAG-3’ |
|
5’-GGTCAGGGGTGGTTATTGC-3’ |
|
Piezo1
|
5’-GGCAACTTCCTCACCAAGA-3’ |
|
5’-GGGTATTTCTTCTCTGTCTCT-3’ |
|
GAPDH
|
5’-CGGGAAACTGTGGCGTGAT-3’ |
|
5’-GTCGCTGTTGAAGTCAGAGGAG-3’ |

Cell proliferation assay
PDLSCs were treated with different concentrations of LPS for 7 days, and cell proliferation was tested using a CCK-8 assay (Beyotime, Shanghai, China). The cells were plated in 96-well plates at an initial density of 2×104 cells/ml and cultured for 24 h. Then, 100 μl of medium containing 10 μl of CCK-8 reagent was added, followed by incubation at 37℃ for 2 h, and the absorbance was measured at 450 nm by a microplate reader (EnSpire, USA). Three parallel replicates were prepared, and the OD values were measured on days 0, 1, 3, 5, and 7.
Tube formation assay
PDLSCs in different groups were cultured for 14 days, exposed to the corresponding experimental conditions, and resuspended in the indicated medium. The cell numbers were counted, and 4×104 cells per dish were planted into 96-well plates covered with Matrigel (Corning, USA) and incubated at 37℃ and 5% CO2 for 12 h. The cells were observed under an inverted microscope (Leica, Germany). Three replicate wells per dish were prepared, and 3 fields of view were selected for each well.
Alizarin red S staining
The formation of mineralized nodules was determined by Alizarin red S staining. A total of 4×105 cells were planted in each dish, and the osteogenic induction medium was used when the cells reached 80∼90% confluence. Then, the cells were cultured for 14 or 21 days, washed with PBS 3 times, and fixed at room temperature in 4% paraformaldehyde (Solarbio, Beijing, China) for 30 min. Subsequently, the cells were washed with distilled water 3 times and then stained with the staining (Solarbio, Beijing, China) solution for 20 min. Three replicate wells per group were prepared, the cells were observed under an inverted microscope, and 3 fields of view were selected for each well.
Oil red O staining
A total of 4×105 cells were planted in dishes, and adipogenic induction medium was used when the cells were 80∼90% confluent. Then, the cells were cultured for 14 or 21 days, washed 3 times with PBS, fixed at room temperature with 4% paraformaldehyde, washed with PBS again, incubated with an oil red O (Solarbio, Beijing, China) solution for 15 min, and washed with PBS 3 times, after which fat droplets were imaged and appeared red. Three replicate wells per group were prepared, the cells were observed under an inverted microscope, and 3 fields of view were selected for each well.
Immunofluorescence staining
Immunofluorescence staining was performed when the cells were cultured in endothelial induction media for 14 days, and then, the expression of CD31 and Piezo1 was examined. A total of 4×105 cells were planted in dish, and when the cells reached 80% confluence, they were cultured in normal medium or induction medium. All cells were cultured in 3.5 cm dishes containing creep plates (Biosharp, Beijing, China). The cells were fixed in 4% paraformaldehyde for 30 min, rinsed twice with PBS, blocked with 1% bovine serum albumin (BSA) (Beyotime, Shanghai, China) for 30 min, incubated overnight at 4℃ with primary antibodies (rabbit or mouse) (Abcam, USA), and then washed three times with PBS. Alexa Fluor 488-labeled goat anti-mouse (Bioworld, USA) or Cy5-labeled goat anti-rabbit (Bioworld, USA) secondary antibodies were then added and incubated for 30 min. Finally, the samples were stained with DAPI (Beyotime, Shanghai, China) in antifading reagent and observed under fluorescence microscope (YC.YX-2050, Olympus, Japan). The control sample was composed of cells that were not incubated with antibodies for background fluorescence assessment. Three replicate wells per group were prepared, and 3 fields of view were selected for each well.
Western blot
A total of 5×105 cells were planted in dishes, induction medium was used when the cells were 80∼90% confluent and then, the cells were cultured for 14 days. Total proteins were extracted from cultured cells using RIPA buffer (Solarbio, Beijing, China). Equal amounts of proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Bio-Rad, USA) and transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Ltd., USA). The PVDF membrane was incubated with primary antibodies (Piezo1 diluted 1:2,000) (Abcam, USA) at 4℃ overnight. After being washed with Tris-buffered saline plus Tween-20 (TBST) (Solarbio, Beijing, China) three times, the PVDF membrane was incubated with the corresponding secondary antibody (1:5,000) (Bioworld, USA), followed by ECL detection (Beyotime, Shanghai, China). ImageJ software (National Institutes of Health, USA) was used to quantify the band intensity obtained by protein imprinting analysis. The signal of each target band was normalized to the signal of the GAPDH band. Three replicate wells per group were prepared.
Statistical analysis
Statistical tests were performed by GraphPad Prism (version 8.0, GraphPad Software, Inc., California, USA). The data are presented as the mean±SD. One-way analysis of variance (one-way ANOVA) was used for multiple group comparisons. A value of *p<0.05 was considered statistically significant. All experiments were independently repeated at least 3 times (n≥3).
Go to :

Discussion
The clinical application of LIPUS has received extensive attention in recent years, and LIPUS can be used in fracture repair, tissue injury healing and blood supply-related diseases. LIPUS promotes not only osteogenic differentiation and bone defect repair but also angiogenesis in HUVECs, which are widely used in the treatment of atherosclerosis and tissue hypoxia.
Compared with mature blood vessels, newly formed vessels, which are immature vascular networks, have distinct characteristics. In addition, blood vessels in different tissues exhibit unique characteristics and functional properties (
18). The periodontal ligament is a connective tissue containing an approximately 20% vascular supply, and PDLSCs are found around the periodontal vascular plexus (
3). PDLSCs not only promote the formation of functional blood vessels when cocultured with HUVECs (
19) but also differentiate into endothelial-like cells, which can form microvessels (
20). These data confirmed that PDLSCs are closely related to the periodontal microvascular network. LIPUS affects the adipogenic and osteogenic differentiation of MSCs (
9) and promotes angiogenesis when human adipose stem cells (hADSCs) and HUVECs are cocultured (
11). The PCR and Alizarin red staining results in our study showed that LIPUS can promote the osteogenic differentiation of PDLSCs in osteogenic induction medium, and the expression of
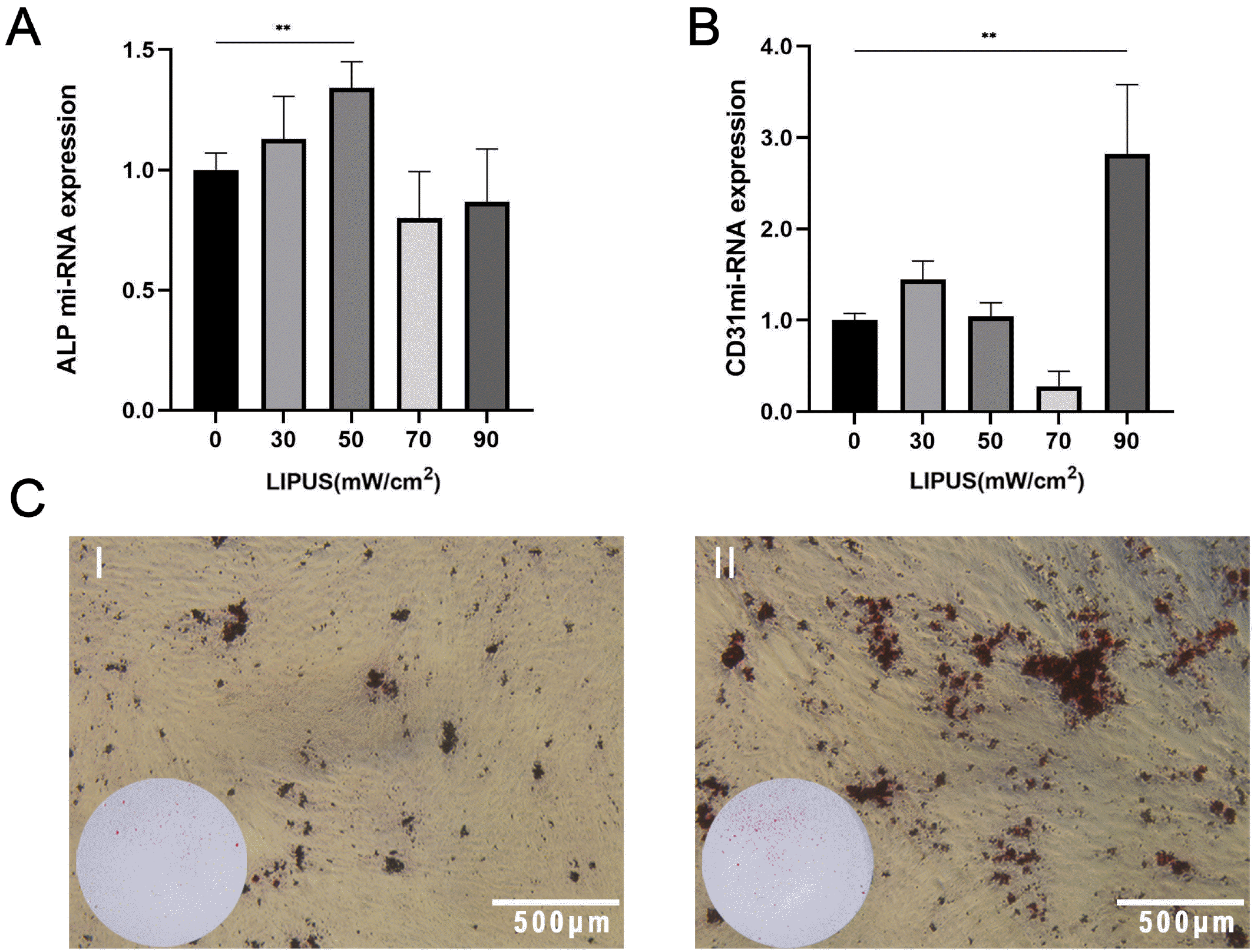
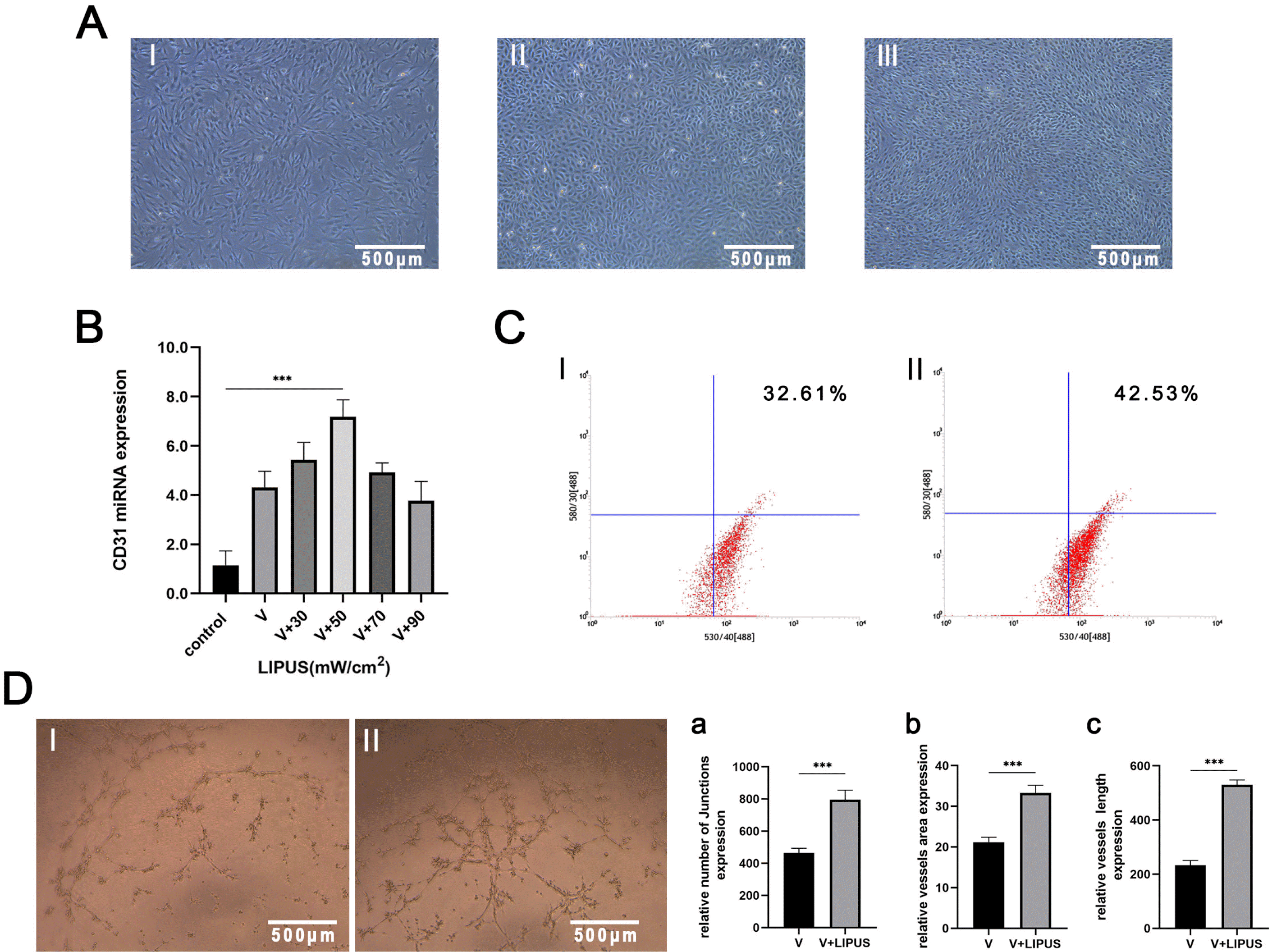
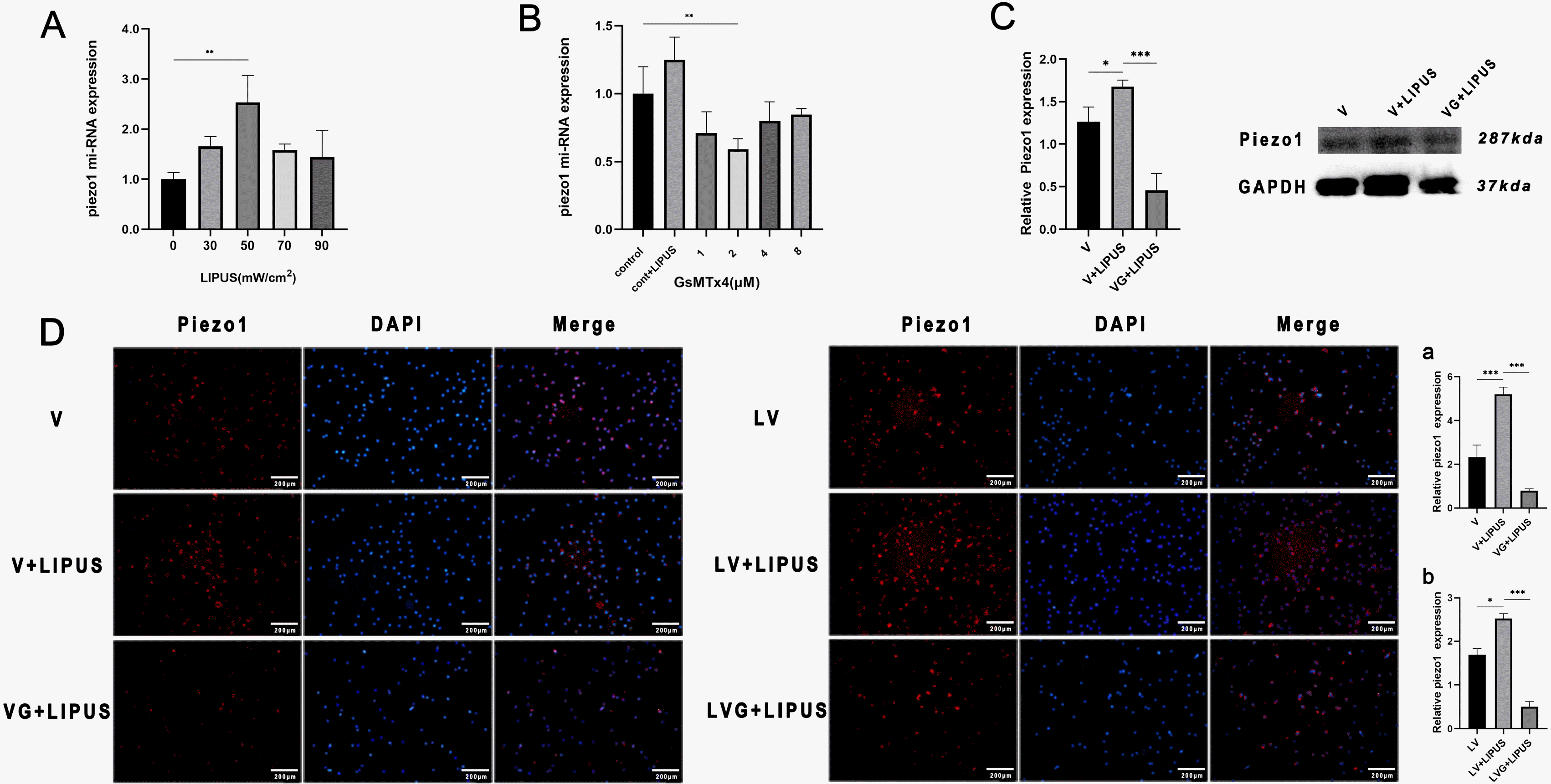
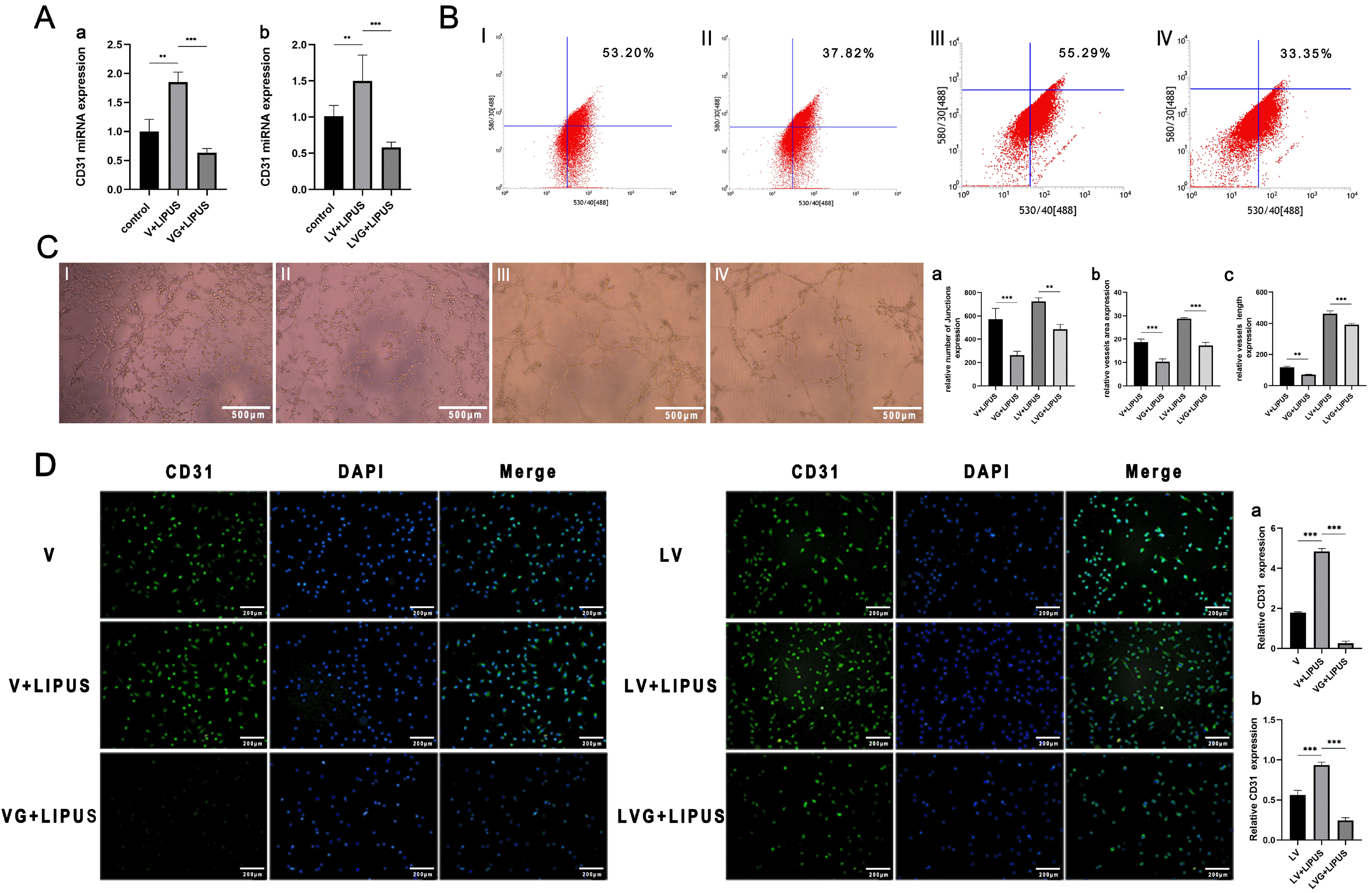
CD31 also increased. Therefore, LIPUS may also affect endothelial differentiation and angiogenesis in PDLSCs. Subsequently, PDLSCs were cultured in endothelial induction medium. The experimental results showed that LIPUS could promote the endothelial differentiation of PDLSCs and the formation of microvessels in differentiated endothelial-like cells. This finding demonstrated that LIPUS not only regulate stem cell differentiation but also affect the tube formation of differentiated endothelial-like cells.
In the early stage of bone repair, intensive capillary networks are needed to promote fracture healing. The formation and regeneration of bone involve angiogenesis and the entry of osteoblast precursors, as well as the invasion of blood vessels into calcified structures that migrate with the growth of blood vessels and osteoclasts. H-type and L-type capillaries link osteogenesis with angiogenesis, and H-type blood vessels mainly support osteogenesis (
6). Existing
in vivo models have shown that H-type blood vessels can promote bone tissue regeneration. In this study,
CD31 was used as an indicator of differentiated endothelial-like cells
in vitro. The results showed that LIPUS significantly increased the
CD31 level in PDLSCs after endothelial differentiation, which is consistent with the results of a previous study
in vivo. In this study, we also found an interesting phenomenon. We treated PDLSCs with LIPUS at different intensities. The sensitivity changes during the differentiation of cells were highly similar to those of PDLSCs cultured in one-way osteogenic induction medium or endothelial induction medium, and the changes were most obvious at an intensity of 50 mW/cm
2. As noted previously, endothelial-like cells differentiated from PDLSCs can form H-type blood vessels, which may be involved in bone formation.
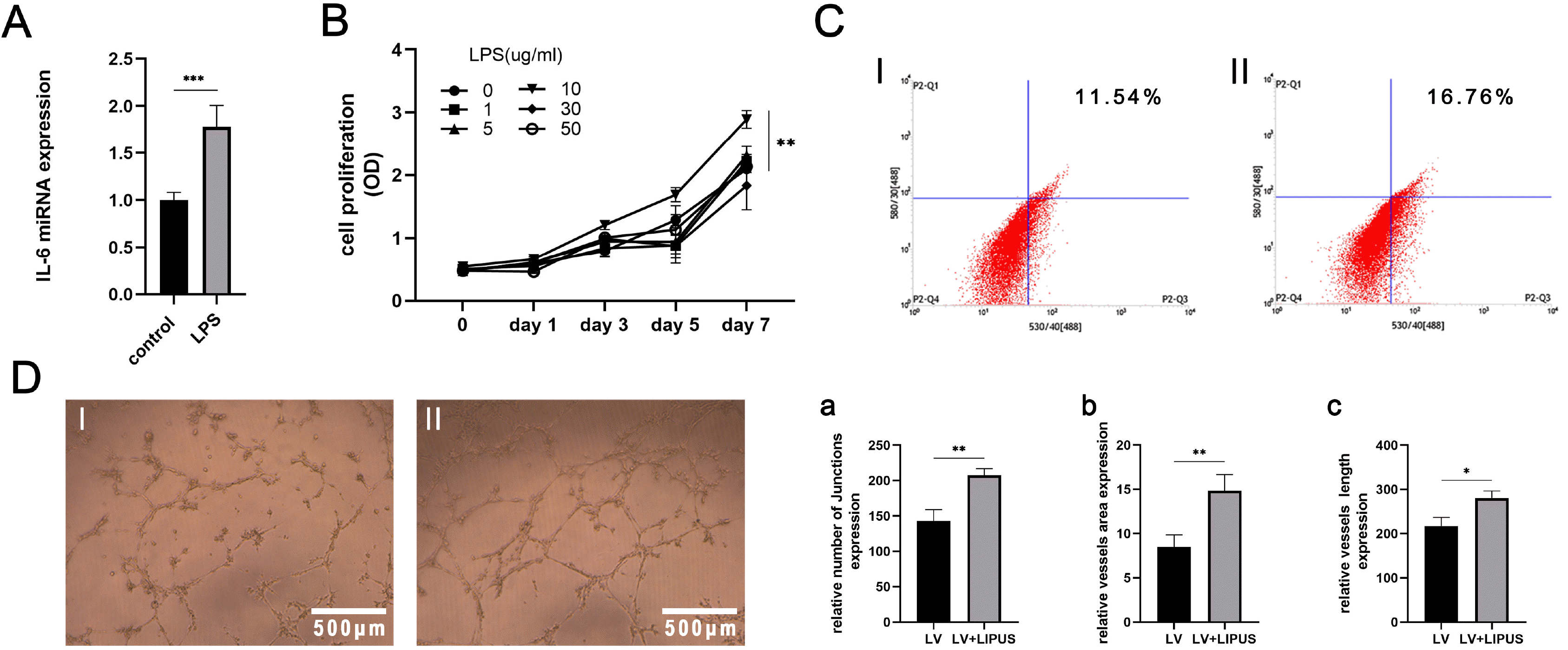
Autophagic activation in PDLSCs in an inflammatory environment promotes angiogenesis in periodontitis, causes abnormal vascularization of the periodontal ligament (
21), and inhibits osteogenic differentiation in PDLSCs (
22). Many studies have shown that the abnormal proliferation of blood vessels and osteogenic differentiation are weakened. We found that the formation of microvessels and osteogenic differentiation occur in the opposite direction, which may be the reason for the formation of granulomas rather than the promotion of osteogenic differentiation in periodontal tissue under inflammatory conditions. Our study showed that LIPUS had positive effects on the inflammatory state, promoted PDLSC differentiation into endothelial-like cells and promoted the formation of microvessels. In addition, LIPUS inhibited autophagy and promoted osteogenic differentiation of PDLSCs in an inflammatory environment (
17). LIPUS may positively affect the negative feedback caused by inflam-mation and also promote microvascular formation and osteogenic differentiation. LIPUS promoted vascularization to provide more nutrients for alveolar bone regeneration. The positive effect of LIPUS may reduce or even prevent the occurrence of abnormal vascularization in inflamed tissues. In addition, infection by periodontal pathogens such as
Porphyromonas gingivalis (P. g.) leads to the rupture of blood vessels by proteases, resulting in local hypoxia. The proportion of anaerobic bacteria in chronic periodontitis is further increased, and the disease is aggravated (
3). Our results showed that LIPUS can promote endothelial differentiation and angiogenesis of PDLSCs in an inflammatory state, which may provide a theoretical basis for LIPUS treatment to alleviate LPS-induced periodontal inflammation (
23) and bone loss. Most of the current treatment methods for periodontitis still involve root scaling to remove root surface bacteria and granulation tissue to create a better microenvironment and then promote wound healing through periodontal tissue regeneration. After clinical curettage, periodontal ligament tissue is damaged to varying degrees, thus affecting its biological function. The repair and regeneration of tissue requires a rich blood supply to provide nutrients. LIPUS treatment is coordinated with clinical surgical treatment to promote osteogenesis and microvascular formation, which is expected to accelerate the repair and regeneration of periodontal tissue.
Mechanically activated ion channels can respond to various physical forces, such as vibration, stretching, acoustic waves (
24) or LIPUS, an ultrasonic radiation force. As a pressure sensor,
Piezo1 is a mechanically sensitive ion channel that can convert the received physical signal into a physiological signal and is expressed in various tissues, including the bladder, colon and lung. Haemodynamic changes can activate the expression of
Piezo1 and regulate angiogenesis-related diseases such as ischemic heart disease, tumors, and atherosclerosis (
25). LIPUS treatment of vascular endothelial cells can activate the expression of
Piezo1 and induce functional changes of vascular endothelial cells (
26). Piezo channels are also abundantly expressed in periodontal tissues and cells and may be involved in the transmission of periodontal mechanical sensation (
27). Thus, LIPUS likely regulates the biological function of PDLSCs by activating
Piezo1. In this study, the
Piezo1 inhibitor of
GsMTx4 was used to inhibit the expression of
Piezo1, and cells were treated with LIPUS with or without
GsMTx4. The experimental results showed that compared with the control group, the
GsMTx4 group showed significantly weakened endothelial differentiation caused by LIPUS and inhibited formation of microvessels. LIPUS may affect intracellular and extracellular material exchange by activating the expression of
Piezo1, thereby promoting endothelial differentiation of PDLSCs and angiogenesis after differentiation.
The clinical application of LIPUS is increasingly widespread, but its specific mechanism is still not clear. In this study, the mechanism by which LIPUS affects the endothelial differentiation of PDLSCs was preliminarily clarified through in vitro experiments, and some theoretical support for the treatment of oral diseases may be provided in the future. The limitation of this study is that it only simulated the regulatory effect of LIPUS on cell differentiation and angiogenesis at the cellular level, and further studies are needed.
In conclusions, in this study, LIPUS promoted the endothelial differentiation and microvascular formation of PDLSCs, and the Piezo1 inhibitor GsMTx4 inhibited the promoting effect of LIPUS. The experimental results indicate that Piezo1 may be involved in the effect of LIPUS on the differentiation and angiogenesis of PDLSCs, and these results need to be further verified in vivo. This study may help to explore the role of LIPUS in clinical periodontal tissue regeneration and the mechanism of PDLSCs in periodontal tissue repair.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download