Abstract
Gastric outlet obstruction is a major symptom in patients with advanced pancreatic cancer. Endoscopic intervention is often challenging in severe strictures because the guidewire cannot pass beyond the stricture. Sometimes, the air itself cannot pass beyond the stricture, which can result in a severely distended stomach. Such a stomach is vulnerable to excessive air insertion or mechanical stress during endoscopic procedures, and endoscopists may encounter a higher rate of complications. Gastric perforation is rare but could be fatal. However, endoscopic management can show a favorable result if the perforation is noticed early. The authors report a case of the perforation of a gastric tear during duodenal stent insertion in a patient with a gastric outlet obstruction.
Go to : 
Gastric outlet obstruction (GOO) is a major symptom encountered in patients with advanced pancreatic cancer. Endoscopic duodenal stenting is safe and effective for palliation, but endoscopic intervention is often challenging for severe stricture as the guidewire could not pass the stricture. Sometimes, air itself is unable to pass beyond the stricture and this can result in a severely distended stomach. Such a stomach is vulnerable to excessive air insertion or mechanical stress during endoscopic procedures, and endoscopists may encounter a higher rate of complications.1 Gastric perforation is rare, but could be fatal. However, if the perforation is noticed early, endoscopic management can show favorable result. Herein, we report a case of perforation of a gastric tear during duodenal stent insertion in a patient with gastric outlet obstruction.
Go to : 
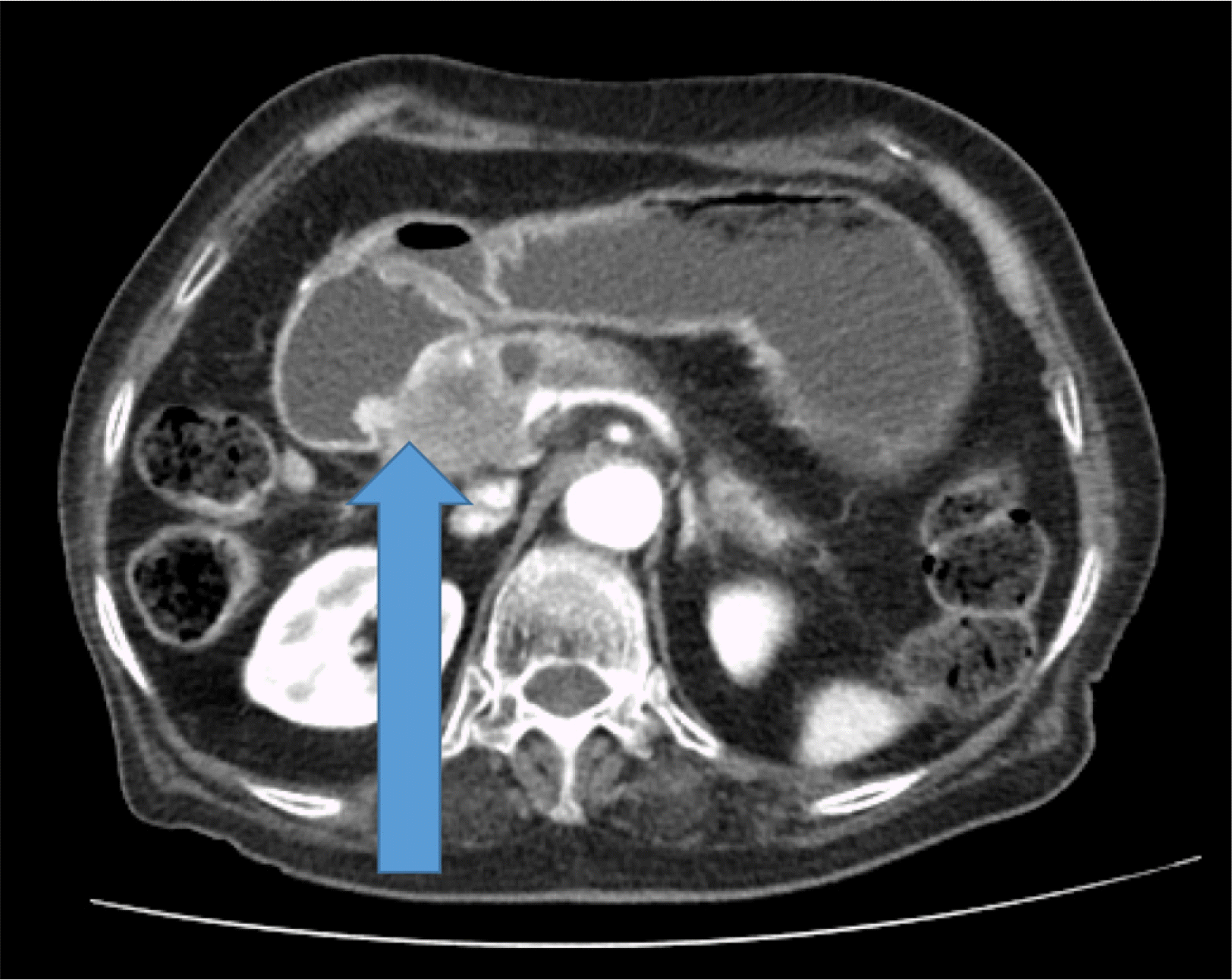
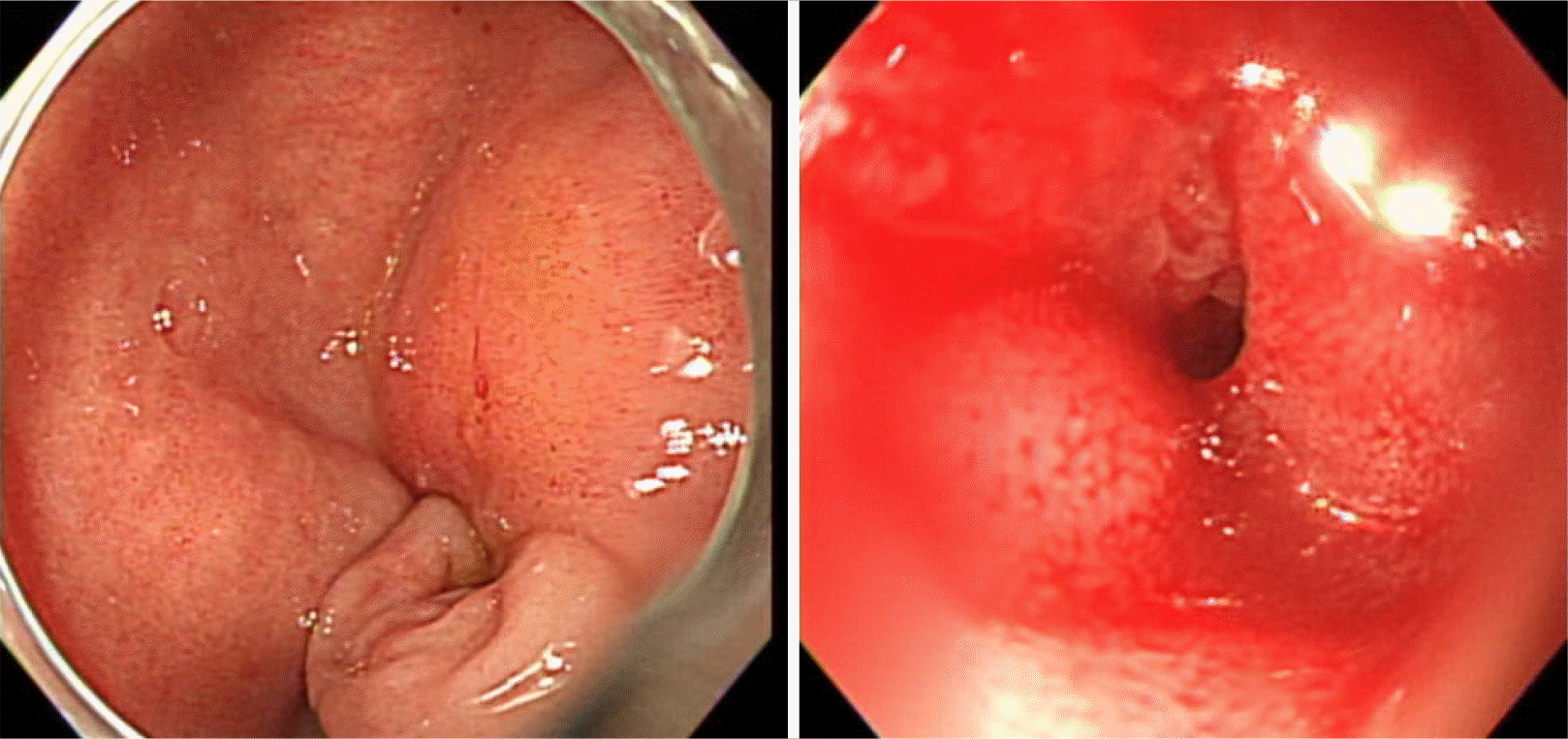
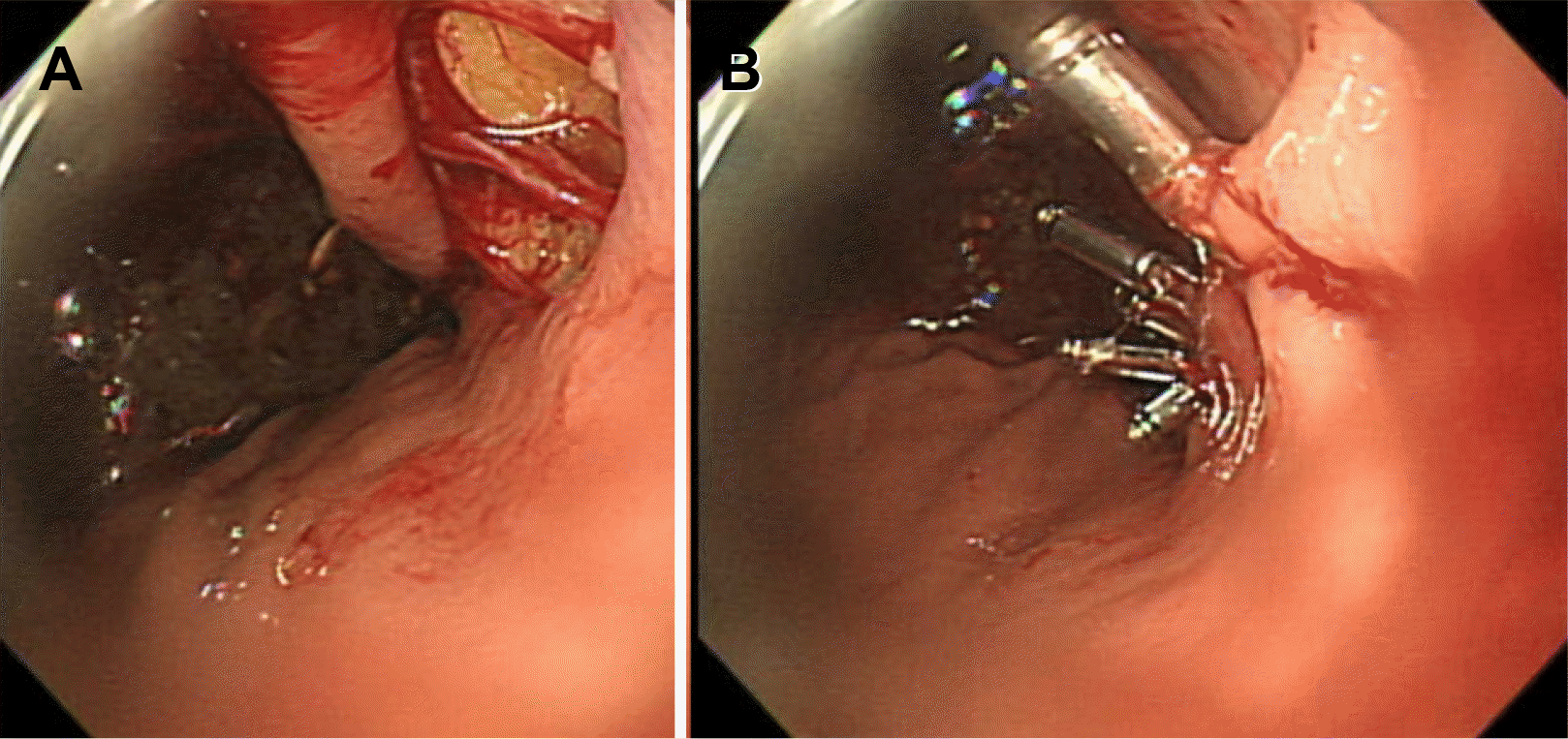
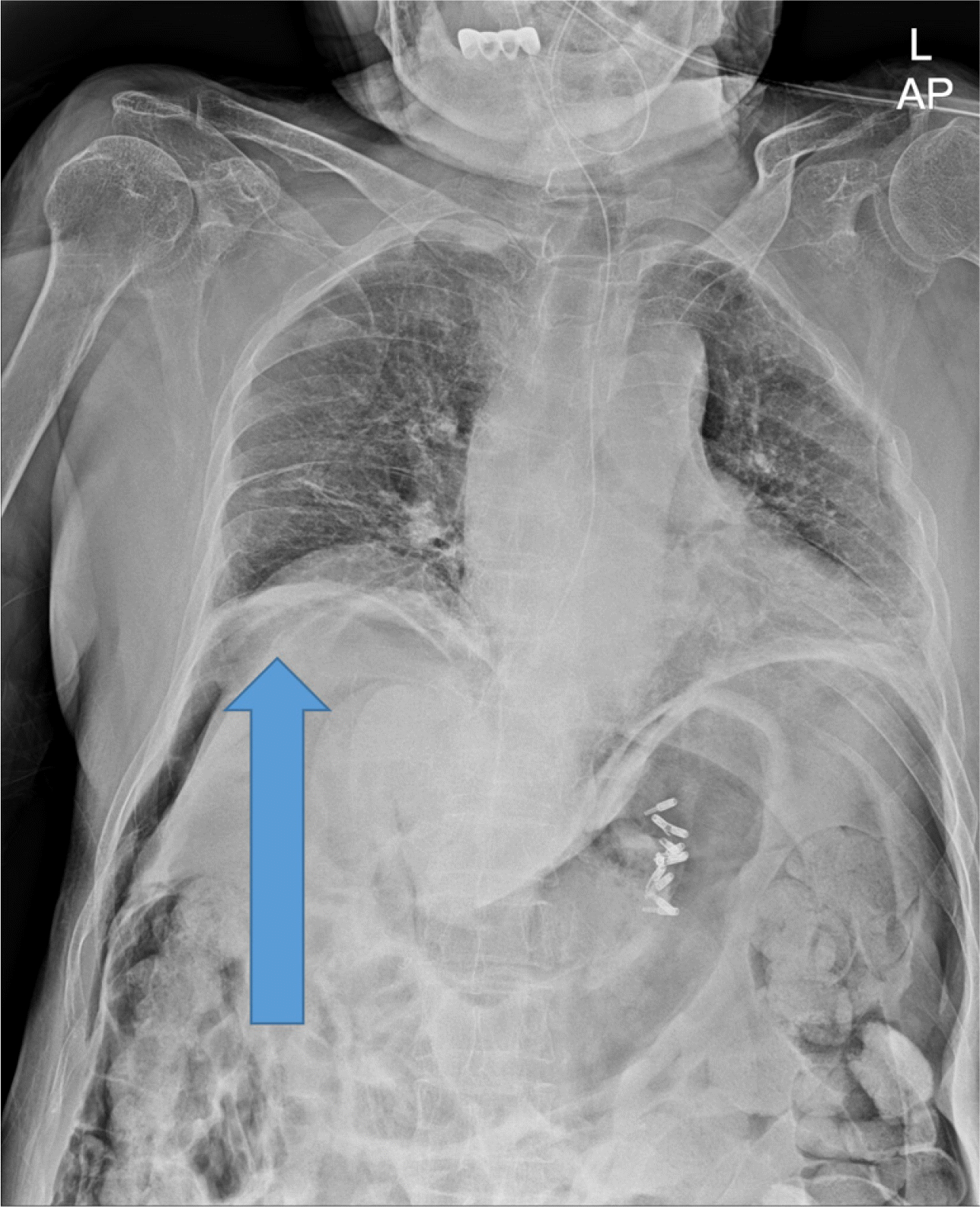
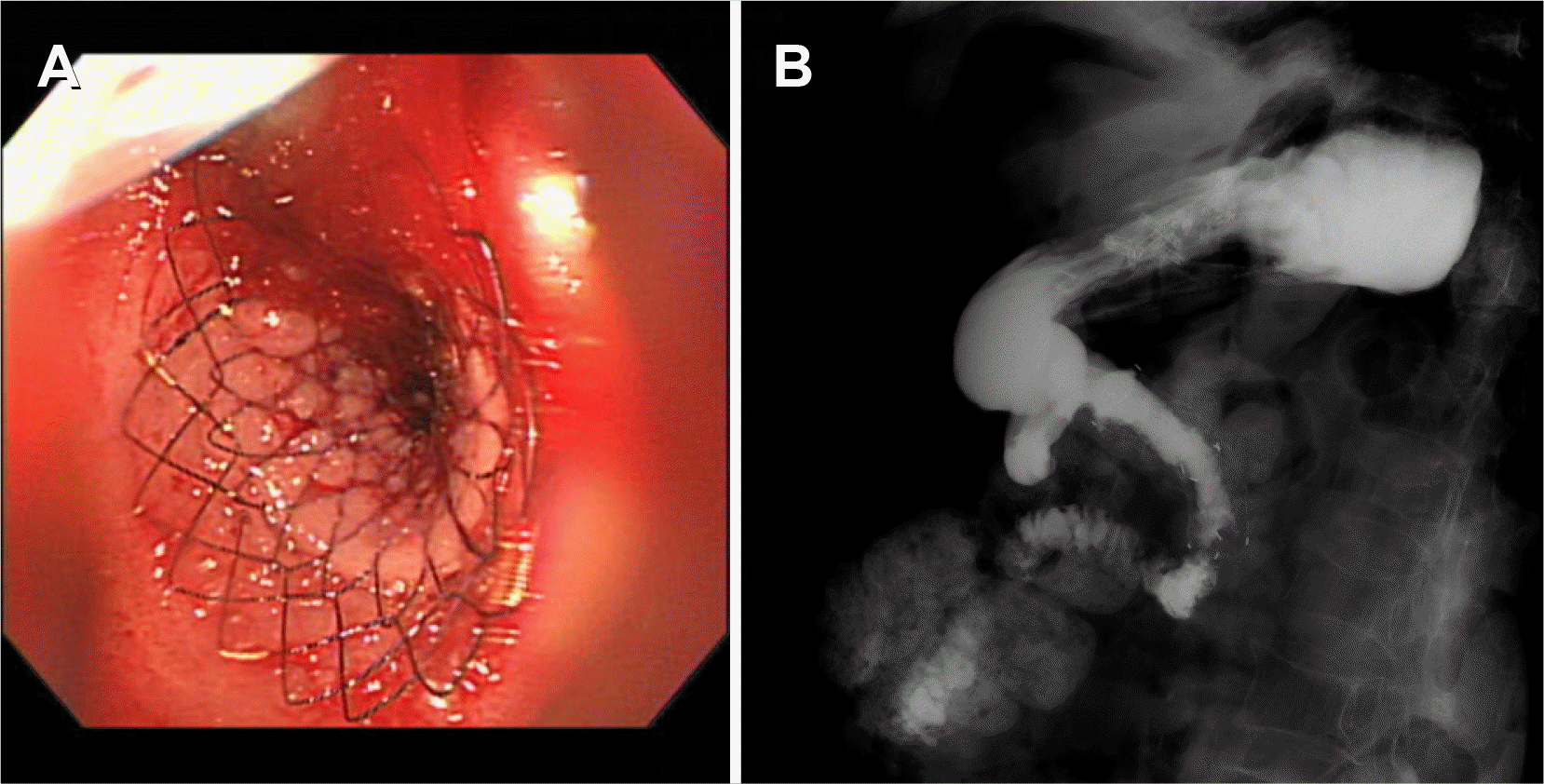
A 91-year-old woman was referred to emergency department of Ulsan University Hospital with a history of weight loss and poor oral intake that had begun 1 month prior to presentation at the hospital. She experienced nausea and vomiting 2 weeks prior and thus had stopped oral intake of food. CT revealed advanced pancreatic cancer with duodenal obstruction (Fig. 1). She underwent a cholecystectomy 30 years previously and was diagnosed with a liver abscess 5 years prior. Endoscopy was performed to evaluate stricture formation and to perform duodenal stenting under fluoroscopy. The patient was sedated using midazolam and pethidine. The stricture was located at the duodenal bulb, and the scope could not pass via the stricture site (Fig. 2). Multiple attempts were made to pass the guidewire through the stricture, but they all resulted in failure. The procedure took approximately 14 minutes. While retrieving the scope, a linear gross perforation approximately 2 cm long was observed at the lesser curvature of the gastric upper body, that revealed the underlying omentum, fluoroscopy revealing pneumoperitoneum (Fig. 3A). The site was immediately closed endoscopically, using endoclips (Fig. 3B). Postsurgical radiography revealed free air in the peritoneum (Fig. 4). The patient did not show signs of peritoneal irritation; therefore, we treated the patient conservatively with Levin tube insertion and intravenous broad-spectrum antibiotics. Air in the peritoneum did not increase for a week, and a follow-up CT scan failed to reveal any macroperforation. Ten days after the perforation was repaired, endoscopy was performed, and revealed that the perforation had healed. Duodenal stenting was performed successfully, and the upper gastrointestinal series of images did not show any leakage from the perforation site (Fig. 5). The patient initiated oral intake of food and was discharged on the 12th day following repair of the perforation.
Go to : 
Gastric perforation or rupture is a rare complication encountered during upper gastrointestinal endoscopy, but one that has the potential to be fatal.2 Mostly, iatrogenic perforations are related to therapeutic procedures themselves, such as endoscopic submucosal dissection (ESD) and gastroenteroic anastomosis dilatation.1 Mechanisms of iatrogenic perforation can be classified as either blunt trauma or electrocoagulation- induced injury.2 Considering the location (lesser curvature of the gastric upper body) and mechanism, rupture of a Mallory-Weiss tear (MWT) is an appropriate diagnosis.3 MWT is a common cause of non-variceal upper gastrointestinal bleeding, characterized by linear, non-perforating mucosal laceration.4,5 Mucosal laceration often occurs during endoscopic examination, but perforation of gastric wall is rare. Boehaave syndrome is a spontaneous perforation of the esophagus resulting from MWT. However, in our case, teared stomach wall was ruptured, which was an uncommon finding in MWT or Boehaave syndrome. The stomach wall had been dilated and weakend for a long time, and air inflation and mechanical stress of endoscopic shaft have resulted in the perforation of the MWT at gastric wall. Esophageal ESD is a known etiology of rupture in MWT.6,7 Similar to esophageal ESD, air suction is not effective as the scope is located in the duodenum. Moreover, duodenal obstruction exacerbates gastric distension by preventing gas from passing through the stricture. Additionally, atrophied stomach walls due to old age and reduced belching under sedation might also have been contributing factors in this case.
Several risk factors have been hypothesized to be associated with gastric perforations (female sex, presence of ulcer or unhealthy tissues, age >80 years, large tumor size, and long duration of resection).1,2 The thinner upper region is the most common site of perforation. Therefore, it is important to pay special attention to this site when retrieving the scope after completing any type of procedure. The management of gastric perforation depends on the timing and size of the defect. Kang et al.2 and Merchea et al.8 reported that 67% and 42% of iatrogenic upper endoscopic perforations were diagnosed during the procedure itself, respectively. If a perforation is diagnosed during the procedure or within 12 hours following it, endoscopic closure can be attempted, with good clinical outcomes.1 If the defect is smaller than 1 cm, endoscopic clipping is an acceptable modality of treatment. If the defect is larger than 10 mm as was the case in our patient, an over-the-scope clip system would be the most appropriate choice.9 When the omentum is visible, and the defect is large, the omental patch technique may be attempted.10 However, such new devices are in limited use. If the diagnosis is late, conservative treatment can be attempted if there is no free fluid or contrast extravasation on the CT evaluation. Timely surgical management is necessary if there are clinical signs of sepsis or peritonitis.
Carbon dioxide (CO2) is used in colonoscopy to prevent gas-related complications, as CO2 is rapidly absorbed by the small bowel. However, using CO2 may not help in cases similar to ours, as the duodenum is obstructed.6 Rather, monitoring gastric volume under fluoroscopy may be helpful. Timely suction and gentle as well as short procedures are key to preventing gas-induced complications.
In conclusion, endoscopists should be aware of gastric overinflation as a complication encountered during duodenal stent insertion in GOO. Gastric gas suction should be performed frequently and excessive mechanical stress should be avoided. Endoscopic clipping and conservative treatment may be effective in managing iatrogenic gastric perforations, provided they are diagnosed promptly.
Go to : 
REFERENCES
1. Paspatis GA, Arvanitakis M, Dumonceau JM, et al. 2020; Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) position statement- update 2020. Endoscopy. 52:792–810. DOI: 10.1055/a-1222-3191. PMID: 32781470.

2. Kang DH, Ryu DG, Choi CW, et al. 2019; Clinical outcomes of iatrogenic upper gastrointestinal endoscopic perforation: a 10-year study. BMC Gastroenterol. 19:218. DOI: 10.1186/s12876-019-1139-1. PMID: 31842778. PMCID: PMC6916018.

3. Okada M, Ishimura N, Shimura S, et al. 2015; Circumferential distribution and location of Mallory-Weiss tears: recent trends. Endosc Int Open. 3:E418–E424. DOI: 10.1055/s-0034-1392367. PMID: 26528495. PMCID: PMC4612247.

4. Kim HS. 2015; Endoscopic management of Mallory-Weiss tearing. Clin Endosc. 48:102–105. DOI: 10.5946/ce.2015.48.2.102. PMID: 25844336. PMCID: PMC4381135.

5. Na S, Ahn JY, Jung KW, et al. 2017; Risk factors for an iatrogenic Mallory-Weiss tear requiring bleeding control during a screening upper endoscopy. Gastroenterol Res Pract. 2017:5454791. DOI: 10.1155/2017/5454791. PMID: 28348579. PMCID: PMC5350415.

6. Yamasaki T, Sakata Y, Suekane T, Nebiki H. 2021; Perforation of a gastric tear during esophageal endoscopic submucosal dissection under general anesthesia. Clin Endosc. 54:916–919. DOI: 10.5946/ce.2020.220. PMID: 33176411. PMCID: PMC8652158.

7. Chen W, Zhu XN, Wang J, Zhu LL, Gan T, Yang JL. 2019; Risk factors for Mallory-Weiss tear during endoscopic submucosal dissection of superficial esophageal neoplasms. World J Gastroenterol. 25:5174–5184. DOI: 10.3748/wjg.v25.i34.5174. PMID: 31558865. PMCID: PMC6747285.

8. Merchea A, Cullinane DC, Sawyer MD, et al. 2010; Esophagogastroduodenoscopy-associated gastrointestinal perforations: a single-center experience. Surgery. 148:876–882. DOI: 10.1016/j.surg.2010.07.010. PMID: 20708766.

9. Baron TH, Song LM, Ross A, Tokar JL, Irani S, Kozarek RA. 2012; Use of an over-the-scope clipping device: multicenter retrospective results of the first U.S. experience (with videos). Gastrointest Endosc. 76:202–208. DOI: 10.1016/j.gie.2012.03.250. PMID: 22726484.

10. Abidi WM, Thompson CC. 2016; Endoscopic closure of an iatrogenic perforation with an omental patch. Gastrointest Endosc. 83:652. DOI: 10.1016/j.gie.2015.09.004. PMID: 26386390.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download