Abstract
Objectives.
Despite the efficacy of surgical treatments, the high rate of recurrence in juvenile nasopharyngeal angiofibroma (JNA) after surgery remains an unresolved problem. The present study comprehensively analyzed the risk factors and characteristics of JNA recurrence, providing clinical guidance for reducing recurrence.
Methods.
A total of 123 patients who underwent surgery for JNA between 1997 and 2019 at a single hospital were analyzed retrospectively. Univariate and multivariate analyses were used to assess the clinical risk factors for the recurrence of JNA. The relapse-free survival and annual cumulative recurrence rates were analyzed for subgroups defined according to clinical parameters.
Results.
After screening, 78 of the 123 patients were included in the present study. The main risk factors associated with JNA recurrence included the year of diagnosis, tumor size, sphenoid bone invasion, Radkowski stage, surgical approach, and intraoperative bleeding. Importantly, the surgical approach and sphenoid bone invasion were independent prognostic factors affecting recurrence. Patients who underwent endoscopic surgery without sphenoid bone invasion exhibited longer relapse-free survival. In the present study, the overall cumulative recurrence rate of JNA was 38.7%, and recurrence occurred mainly in the first year after the initial surgery.
Conclusion.
Endoscopic surgery achieved better relapse-free survival in JNA patients, and patients with sphenoid bone invasion should be carefully explored to avoid residual JNA. The recurrence rate of JNA differed among subgroups defined based on clinical parameters and was highest in the first year after surgery. Computed tomography or magnetic resonance imaging, along with close follow-up, should be performed strictly within 1 year after the primary operation.
Juvenile nasopharyngeal angiofibroma (JNA) is a rare benign vascular tumor that accounts for 0.05% to 1% of head and neck tumors [1,2]. JNA occurs almost exclusively in adolescent males, with nasal bleeding and nasal obstruction as the main clinical symptoms [3]. Although it is not a malignant tumor and does not metastasize, its highly invasive behavior and locally aggressive growth pattern make it prone to life-threatening hemorrhage [4,5]. JNA can invade the nasopharynx, paranasal sinus, pterygopalatine fossa, sphenoid bone, and even the base of the skull [6]. Surgery is the preferred treatment for JNA, including open, endoscopic, or combined surgical approaches.
Despite the highly effective surgical treatments for JNA, with favorable survival outcomes, the high rate of recurrence after surgery remains one of the greatest challenges for otolaryngologists [7]. Studies have reported that the recurrence rates of JNA vary from approximately 20% to over 50% [8,9]. Due to the biological behavior of the tumor, surgery usually fails to completely remove it, particularly for advanced tumors, which contributes to the tumor residue and is the main cause of postoperative recurrence [10]. Tumors invading the infratemporal fossa, cavernous sinus, pterygoid canal, and sphenoid bone have been thought to have a higher recurrence rate because tumor residue occurs very easily [7]. Moreover, increasingly many studies have suggested that the clinicopathological characteristics or treatment options could affect postoperative recurrence [11]. However, the effect of preoperative embolization on postoperative recurrence remains a matter of debate [12-14]. Other studies have reported that the risk factors for JNA recurrence included tumor stage [15] and tumor size [16]. However, most studies on relapse were limited to a single risk factor or a small number of cases, lacking a comprehensive and systematic analysis. It is crucial to explore the clinical risk factors to predict JNA recurrence and reduce the recurrence rate.
Based on a retrospective analysis of 123 cases over the past 22 years, the present study sought to (1) analyze the clinical risk factors for JNA recurrence and compare the risk of recurrence among subgroups defined based on clinical parameters (2), characterize annualized JNA recurrence rates overall and among clinical parameter-based subgroups, and (3) describe the characteristics of JNA recurrence sites for each anatomical location.
This study was a retrospective study and approved by the First Affiliated Hospital of Sun Yat-sen University. Our study did not require informed patient consent because we only collected the patients hospitalization data but did not perform any invasive procedures on the patients.
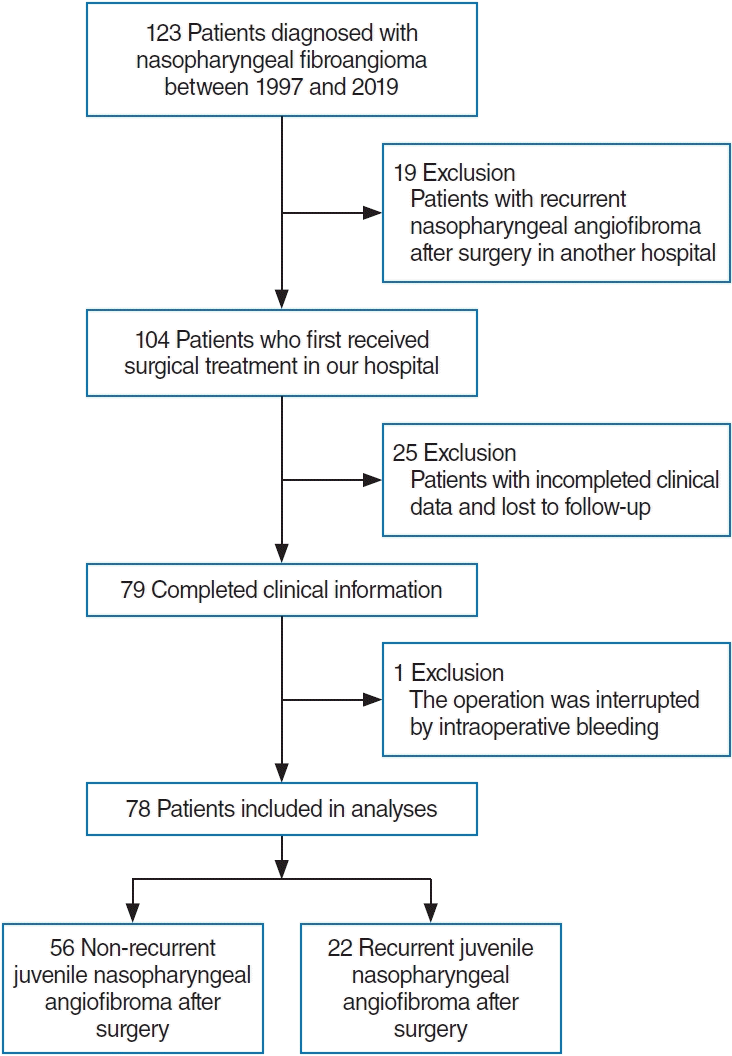
The clinical parameters of 123 patients who were pathologically diagnosed with JNA and received surgical treatment at Hospital between 1997 and 2019 were collected for further screening. Patients who met the following criteria were included in our retrospective study: (1) patients undergoing initial surgical treatment in our hospital but without radiation or hormone therapy; (2) patients who had completed clinical information, including age at diagnosis, year of diagnosis, tumor size, Radkowski stage, tumor location, presence of preoperative embolization, surgical approach and presence of intraoperative bleeding; (3) the surgical treatment was not a secondary staging operation; and (4) complete follow-up information. The flowchart for study cohort selection is shown in Fig. 1. Preoperative computed tomography (CT) or magnetic resonance imaging (MRI) scans were adopted to classify the tumor site and determine the tumor stage according to the Radkowski staging system [17]. We considered tumor invasion of the pterygoid process, pterygoid plates or sphenoid body as sphenoid invasion; if none of these conditions were met, there was determined to be no sphenoid invasion. With the vigorous promotion and development of nasal endoscopic surgery in our hospital after 2010, more patients with JNA preferred to choose the nasal endoscopic surgery, and early postoperative CT and MRI were beginning to be performed for checking the residual lesions. Patients were followed up to February 5, 2021; for patients who were lost to follow-up, the last follow-up time was used as the endpoint. The occurrence of residue/recurrence was evaluated according to examination of postoperative CT or MRI imaging and patient symptoms.
Initially, chi-square and Fisher’s exact tests were performed to compare the influence of clinicopathological features between patients with and without recurrence. The unpaired t-test was used to compare the different clinicopathological features on intraoperative blood loss. Next, the Kaplan-Meier method and a log-rank test were used to evaluate the recurrence-free survival rate of each clinical parameter. Subsequently, the risk factors for recurrence of JNA were analyzed by univariate and multivariate analyses using the Cox proportional hazards model with a stepwise backward regression. We also calculated the 1-year, 2-year, 3-year, 5-year and overall cumulative incidence rate of JNA recurrence using the Cox proportional hazard regression analysis. Finally, the frequency of anatomical sites of postoperative recurrence for patients within our hospital and other hospitals was analyzed.
IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7 (San Diego, CA, USA) were used for all statistical analyses in this article. The statistical values were expressed as mean±standard error (SE). The results from two-sided tests with a P<0.05 were considered statistically significant, and a P<0.01 was considered very significant.
In total, 123 patients were diagnosed with JNA between 1997 and 2019, and their clinical parameters were collected. Seventy-eight patients aged 10–29 years (16.5±4.4 years) were screened for inclusion in this retrospective analysis (Fig. 1). There were no statistically significant differences in tumor stage and surgical method between before and after 2010 (P<0.05) (Supplementary Table 1). However, we found that patients were mainly stage II (55.17%) after 2010, while patients with stage I (45.00%) were predominantly present before 2010. Moreover, it was clear that patients more frequently preferred to choose endoscopic surgery (45.00% vs. 67.24%) instead of open surgery (20.00% vs. 5.17% after 2010 than before 2010.
Over a median follow-up of 31 months, 22 patients (28.21%) experienced JNA recurrence, while 56 patients (71.79%) had no recurrence. Using the median age as the cutoff point, we divided patients into groups of >15 years and ≤15 years. Based on the Radkowski stage classification, there were 24 (30.77%) patients with stage Ia–Ib, 38 (48.72%) with stage IIa–IIc and 16 (20.51%) with stage IIIa–IIIb disease. Of the 41 patients treated with endoscopic surgery, seven (17.01%) developed recurrence, whereas four (57.14%) of the seven patients treated with open surgery experienced recurrence.
Comparing the clinical parameters for patients with and without recurrence, statistically significant differences were observed for the year at diagnosis (P<0.03), tumor size (P<0.01), sphenoid bone invasion (P<0.01), Radkowski stage (P<0.01), surgical approach (P<0.01) and intraoperative bleeding (P<0.01), but there was no significant difference between the groups for age at diagnosis (P<0.08) and preoperative embolization (P<0.11). Moreover, the relapsed patients had a shorter median follow-up time than the non-relapsed patients (median follow-up time: 6.6 months vs. 63.1 months, respectively; P<0.01) (Table 1).
Furthermore, analyzing the effects of clinical characteristics on intraoperative bleeding (Supplementary Fig. 1), we found that patients with tumor size >4 cm had significantly more intraoperative bleeding than those with tumor size ≤4 cm (1,558.0±323.8 mL vs. 629.2±121.3 mL, P<0.01) (Supplementary Fig. 1A). Compared to the patients with stage III, the patients with earlystage disease (stage I) had significantly less intraoperative bleeding (1,875.0±421.3 mL vs. 673.3±214.2 mL, P<0.01) (Supplementary Fig. 1B). The surgical approach was significantly associated with the intraoperative blood loss; specifically, patients who underwent endoscopic surgery had less intraoperative blood loss than those who received open and combined endoscopic and open surgery P<0.05) (Supplementary Fig. 1D). However, whether patients were treated with preoperative embolization had no significant effect on intraoperative blood loss (P<0.05) (Supplementary Fig. 1C). In addition, preoperative embolization was associated with the tumor stage, but not tumor size (Supplementary Table 2). Therefore, preoperative embolization would be preferable in patients with advanced stage (stage I: 37.5%, stage II: 52.63%, stage III: 81.25%; P<0.05).
The results of the univariate Cox hazard regression analysis showed that year at diagnosis, tumor size, sphenoid bone invasion, Radkowski stage, surgical approach and intraoperative bleeding were factors impacting recurrence (Table 2). Patients with tumor size >4 cm (hazard ratio [HR], 5.48; 95% confidence interval [CI], 1.85–16.25; P<0.01), with stage III disease (HR, 4.89; 95% CI, 1.84–25.34; P<0.03), with lesions involving the sphenoid bone (HR, 4.00; 95% CI, 1.68–9.57; P<0.01), who received open surgery (HR, 3.75; 95% CI, 1.09–12.92; P<0.04) or combined endoscopic and open surgery (HR, 4.31; 95% CI, 1.66–11.16; P<0.01), and patients with intraoperative bleeding ≥800 mL had a higher risk of recurrence, whereas patients diagnosed after 2010 (HR, 0.40; 95% CI, 0.17–0.95; P<0.04) had a lower rate of recurrence. Further analysis based on a multivariable logistic model showed that year at diagnosis, sphenoid bone invasion and surgical approach were independent predictors of JNA relapse (Table 2). Patients with sphenoid bone involvement had a 6.09-fold (95% CI, 1.61–23.03) higher recurrence risk than those without sphenoid bone involvement. Open surgery was associated with a significant 4.10-fold (95% CI, 1.60–10.53) elevation in JNA recurrence risk compared with endoscopic surgery, whereas combined endoscopic and open surgery was associated with a 4.55-fold (95% CI, 1.62–12.75) higher recurrence risk. Furthermore, patients after 2010 had a lower risk of relapse (HR, 0.29; 95% CI, 0.11–0.73; P<0.01).
The results of the Kaplan-Meier survival analysis showed that patients who were diagnosed after 2010 (P<0.03), had a tumor size ≤4 cm (P<0.01), had no sphenoid bone involvement (P<0.01), had early Radkowski stage disease (P<0.01), received endoscopic surgery (P<0.01) and had intraoperative bleeding <800 mL had longer recurrence-free survival (Table 2). JNA patients who underwent endoscopic surgery and experienced reduced intraoperative bleeding exhibited a survival benefit.
Table 3 clearly shows that the recurrence of JNA occurred mainly in the first year after surgery (16/78), and the cumulative incidence of JNA recurrence did not change significantly after 2 years. The 1-year, 2-year, 3-year, and 5-year cumulative incidence rates of JNA recurrence in the overall cohort were 21.8%, 26.3%, 28.0%, and 29.9%, respectively, with an overall cumulative incidence of JNA recurrence of 38.7% (Table 3). An analysis of the cumulative recurrence rates of subgroups with different clinicopathological parameters revealed that the 5-year cumulative incidence of JNA recurrence was highest among patients aged ≤15 years (38.6%), diagnosed before 2010 (46.9%), with tumor size >4 cm (48.0%) and with sphenoid bone invasion (55.2%) compared with patients age >15 years (20.5%), diagnosed after 2010 (24.0%), with tumor size ≤4 cm (11.7%), and with no sphenoid bone invasion (12.9%) (Table 3). Patients with advanced-stage disease had a 5-year cumulative incidence rate of 31.1% (stage IIa–IIc) or 58.0% (stage IIIa–IIIb), whereas the rate was 12.5% for patients with Radkowski stage Ia-Ib. Moreover, patients who received open surgery (42.9%) or combined endoscopic and open surgery (51.0%) had higher 5-year cumulative incidence rates of JNA recurrence than patients who underwent endoscopic surgery (17.5%). Similar trends in the cumulative incidence rates were observed between preoperative embolization and no preoperative embolization groups (13.4%) and patients with intraoperative bleeding ≥800 mL (55.6%) versus <800 mL (15.6%).
There were 41 JNA patients (22 patients underwent initial surgery in our center, while 19 patients underwent initial surgery at another center and a second procedure for recurrence at our center) who experienced recurrence after the initial surgery. The frequency and proportion of recurrence at each anatomical site, based on CT scans, are shown in Table 4. The nasopharynx (70.73%), pterygopalatine fossa (63.41%), and sphenoid bone (51.22%) were the most frequent anatomical sites of JNA recurrence, regardless of whether the initial operation was performed at our center or another center. These findings indicate that lesions invading the nasopharynx, pterygopalatine fossa, and sphenoid bone should be carefully and thoroughly removed to avoid JNA residue.
JNA is a rare, nonencapsulated vascular tumor that tends to result in a postoperative residue and has a high recurrence rate [18]. Its pathogenesis is still unclear, and a vascular malformation caused by non-absorption of artery residues in the first branchial arch during development, as well as hormonal and genetic factors, are considered to be involved in its etiology [19].
According to the literature, the recurrence rates of JNA range from 5% to 55% [20]. Our study showed that 22 of 78 patients experienced recurrence, and the overall cumulative recurrence rate was 38.7%. Moreover, the recurrence of JNA mainly occurred during the first year after primary surgical treatment. Therefore, follow-up with CT and/or MRI for JNA patients should be a priority in the first year after surgery.
The present study showed that the year at diagnosis, tumor size, sphenoid bone invasion, Radkowski stage, surgical approach, and intraoperative bleeding were the main risk factors for JNA recurrence, among which the year at diagnosis, sphenoid bone invasion, and surgical approach were independent risk factors. Prior studies also have revealed that the rates of recurrence varied according to the year of diagnosis [21] or even between different centers [22,23]. Over the long time span of this study, we analyzed the recurrence of JNA in different time periods and found that after 2010, significantly fewer patients had postoperative residual disease or recurrence, and the relapse-free survival rate gradually improved. The cumulative recurrence rate of JNA decreased from 55.7% to 24.0% after 2010. This change might be attributed to advances in research on JNA and gradual improvements in surgical techniques. A study at a tertiary center showed that the overall recurrence rate was 39.2% from 1997 to 2006 [16], whereas it was 16.8% from 2006 to 2015, which is considered to be due to the use of endoscopic surgery [10]. Therefore, it is crucial to reduce the recurrence rate by deepening the understanding of JNA, improving surgical skills, and striving for accurate and complete tumor resection.
Due to the complexity of JNA tumor invasion sites, larger tumors and advanced stage may affect the amount of intraoperative blood loss and surgical field of vision, increasing the difficulty of complete tumor resection. Herein, we revealed that patients with tumor size >4 cm exhibited shorter relapse-free survival, and tumor size independently predicted recurrence, which is consistent with previous research [16]. The Radkowski stage, based on the extent of invasion, is another important risk factor for tumor recurrence [10]. Our study showed that most patients who relapsed were at Radkowski stage II or III. An early stage was closely associated with longer relapse-free survival and a lower cumulative recurrence rate (stage I, 12.5%; stage II, 31.1%; stage III, 100%). Regardless, the Radkowski stage was not identified as an independent prognostic factor for JNA recurrence.
The origin of nasopharyngeal angiofibroma is still unclear. Studies have suggested that the invasion of the medial periosteum of the sphenoid body and the pterygoid process are the main reasons for which tumors cannot be completely resected, and have reported that patients with invasion of the base of the pterygoid process had a recurrence rate of 93%, while those without sphenoid bone invasion had a recurrence rate of only 7% [24]. This is consistent with the results of our study, in which patients with tumors invading the sphenoid bone were significantly more likely to develop recurrence and had shorter relapse-free survival than patients without sphenoid bone invasion. Sphenoid bone involvement can be an independent risk factor for JNA recurrence and can predict the recurrence rate. The cumulative recurrence rates of patients with and without sphenoid bone involvement were 55.2% and 29.4%, respectively. In addition, our study revealed that the sphenoid bone is a common anatomical site for recurrence. This suggests that otolaryngologists should pay considerable attention to sphenoid bone invasion during surgery, and complete resection of the tumor in the sphenoid bone should be performed if possible to reduce the risk of residual or recurrent JNA.
In recent years, with gradual improvements in surgical techniques and instruments, the surgical treatment of JNA has progressed from traditional open surgery to endoscopic surgery. A growing number of studies have suggested that endoscopic surgery not only greatly reduces intraoperative bleeding but also has a lower rate of JNA recurrence than open surgery [23,25,26]. This is consistent with the results of our study, which showed that patients who underwent endoscopic surgery had a lower risk of recurrence than those who underwent open surgery or combined endoscopic and open surgery. The incidence of recurrence in patients who received endoscopic surgery was 17.5% compared with 71.4% in the open surgery group, whereas the incidence in the combined endoscopic and open surgery group was 51.0%. This difference could be attributed to the clear visual field and reduced bleeding during the endoscopic operation, which reduced the likelihood of JNA residue. In addition, endoscopic surgery is mostly performed on patients with early-stage disease, whereas advanced patients with large tumors are more likely to undergo open surgery or combined endoscopic and open surgery. This suggests that endoscopic treatment is more beneficial to patients with early-stage disease than to some advanced-stage patients.
Histologically, JNA is an abundantly vascular tissue and has a high rate of hemorrhage [22]. Intraoperative bleeding is another risk factor posing challenges for otolaryngologists. The surgical treatment of advanced, large tumors with deep locations is often interrupted by massive intraoperative bleeding, potentially causing incomplete resection [27]. In the present study, we found that patients with tumor size >4 cm, advanced stage, and those who underwent open or combined endoscopic and open surgery presented significant associations with higher intraoperative blood loss. Moreover, patients who had intraoperative bleeding less than 800 mL exhibited longer relapse-free survival than those who had intraoperative bleeding ≥800 mL. As is well known, preoperative embolization is another important treatment for JNA that can reduce intraoperative bleeding. However, there was no significant difference in relapse-free survival between patients with and without preoperative embolization, which may have been because the majority of patients with preoperative embolization were at an advanced stage. We believe that controlling intraoperative bleeding is the key to ensuring complete surgical resection and a reduced recurrence rate.
There are several limitations of the present study. First, the study spanned a long time frame, which could have led to biases caused by different treatment concepts and surgical techniques. Second, there could have been bias in that symptoms may have been used to assess recurrence in patients without postoperative CT or MRI. Third, patients diagnosed before 2010 were more likely to be lost to follow-up, potentially causing passive selection bias. Finally, we did not evaluate the impact of the surgical approach on recurrence in patients with the same Radkowski stage due to the small number of patients undergoing open surgery in the present study.
▪ In this retrospective analysis, we systematically determined that the surgical approach and sphenoid invasion were independent prognostic factors for the recurrence of juvenile nasopharyngeal angiofibroma (JNA).
▪ Patients who received endoscopic surgery and had no tumor invasion of the sphenoid bone were less likely to have JNA recurrence.
▪ More attention should be paid to complete excision of the tumor invading the sphenoid bone.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found online at https://doi.org/10.21053/ceo.2022.01053.
Supplementary Table 1.
Clinical characteristics of JNA patients before and after 2010
Supplementary Table 2.
Treatment of preoperative embolization among patients with different tumor sizes and stages
Supplementary Fig. 1.
The effects of tumor size (A), Radkowski stage (B), preoperative embolization (C), and surgical approach (D) on intraoperative
bleeding. NS, not significant. *P<0.05, **P<0.01.
ACKNOWLEDGMENTS
The study was supported by the Guangzhou Science and Technology Project of China grant (201704020098 to WW) and the Natural Science Foundation of China grants (81602365, 81972527 to WS).
We thank American Journal Experts from Durham, NC, USA, for editing the English text of a draft of this manuscript.
REFERENCES
1. Lund VJ, Stammberger H, Nicolai P, Castelnuovo P, Beal T, Beham A, et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl. 2010; Jun. 22:1–143.
2. Lao WP, Lagabon KJ, Arom GA, Walker PC, Lee SC. Combined endoscopic and transoral resection of a high-staged juvenile nasopharyngeal angiofibroma: a pictorial essay. Head Neck. 2021; Feb. 43(2):719–24.

3. Nicolai P, Schreiber A, Bolzoni Villaret A. Juvenile angiofibroma: evolution of management. Int J Pediatr. 2012; 2012:412545.

4. Makhasana JA, Kulkarni MA, Vaze S, Shroff AS. Juvenile nasopharyngeal angiofibroma. J Oral Maxillofac Pathol. 2016; May-Aug. 20(2):330.

5. Duvall AJ 3rd, Moreano AE. Juvenile nasopharyngeal angiofibroma: diagnosis and treatment. Otolaryngol Head Neck Surg. 1987; Dec. 97(6):534–40.

6. Hackman T, Snyderman CH, Carrau R, Vescan A, Kassam A. Juvenile nasopharyngeal angiofibroma: the expanded endonasal approach. Am J Rhinol Allergy. 2009; Jan-Feb. 23(1):95–9.

7. Howard DJ, Lloyd G, Lund V. Recurrence and its avoidance in juvenile angiofibroma. Laryngoscope. 2001; Sep. 111(9):1509–11.

8. Fyrmpas G, Konstantinidis I, Constantinidis J. Endoscopic treatment of juvenile nasopharyngeal angiofibromas: our experience and review of the literature. Eur Arch Otorhinolaryngol. 2012; Feb. 269(2):523–9.

9. Bleier BS, Kennedy DW, Palmer JN, Chiu AG, Bloom JD, O’Malley BW Jr. Current management of juvenile nasopharyngeal angiofibroma: a tertiary center experience 1999-2007. Am J Rhinol Allergy. 2009; May-Jun. 23(3):328–30.

10. Liu Z, Hua W, Zhang H, Wang J, Song X, Hu L, et al. The risk factors for residual juvenile nasopharyngeal angiofibroma and the usual residual sites. Am J Otolaryngol. 2019; May-Jun. 40(3):343–6.

11. Pamuk AE, Ozer S, Suslu AE, Akgoz A, Onerci M. Juvenile nasopharyngeal angiofibroma: a single centre’s 11-year experience. J Laryngol Otol. 2018; Nov. 132(11):978–83.

12. Tan G, Ma Z, Long W, Liu L, Zhang B, Chen W, et al. Efficacy of preoperative transcatheter arterial embolization for nasopharyngeal angiofibroma: a comparative study. Cardiovasc Intervent Radiol. 2017; Jun. 40(6):836–44.

13. Desarda KK, Bora MP. Importance of pre-operative embolization in the surgery of nasopharyngeal angiofibroma. Indian J Otolaryngol Head Neck Surg. 1998; Jan. 50(1):36–9.

14. Gargula S, Saint-Maurice JP, Labeyrie MA, Eliezer M, Jourdaine C, Kania R, et al. Embolization of internal carotid artery branches in juvenile nasopharyngeal angiofibroma. Laryngoscope. 2021; Mar. 131(3):E775–80.

15. Dabholkar JP. Juvenile nasopharyngeal angiofibroma: clinical factors associated with recurrence and proposal of a staging system. J Surg Oncol. 2008; Aug. 98(2):73.

16. Sun XC, Wang DH, Yu HP, Wang F, Wang W, Jiang JJ. Analysis of risk factors associated with recurrence of nasopharyngeal angiofibroma. J Otolaryngol Head Neck Surg. 2010; Feb. 39(1):56–61.
17. Radkowski D, McGill T, Healy GB, Ohlms L, Jones DT. Angiofibroma: changes in staging and treatment. Arch Otolaryngol Head Neck Surg. 1996; Feb. 122(2):122–9.

18. Carrillo JF, Albores O, Ramirez-Ortega MC, Aiello-Crocifoglio V, Onate-Ocana LF. An audit of nasopharyngeal fibromas. Eur J Surg Oncol. 2007; Jun. 33(5):655–61.

19. Li W, Ni Y, Lu H, Hu L, Wang D. Current perspectives on the origin theory of juvenile nasopharyngeal angiofibroma. Discov Med. 2019; Jun. 27(150):245–54.
20. Roche PH, Paris J, Regis J, Moulin G, Zanaret M, Thomassin JM, et al. Management of invasive juvenile nasopharyngeal angiofibromas: the role of a multimodality approach. Neurosurgery. 2007; Oct. 61(4):768–77.
21. Mishra A, Mishra SC. Time trends in recurrence of juvenile nasopharyngeal angiofibroma: experience of the past 4 decades. Am J Otolaryngol. 2016; May-Jun. 37(3):265–71.

22. Liu L, Wang R, Huang D, Han D, Ferguson EJ, Shi H, et al. Analysis of intra-operative bleeding and recurrence of juvenile nasopharyngeal angiofibromas. Clin Otolaryngol Allied Sci. 2002; Dec. 27(6):536–40.

23. Boghani Z, Husain Q, Kanumuri VV, Khan MN, Sangvhi S, Liu JK, et al. Juvenile nasopharyngeal angiofibroma: a systematic review and comparison of endoscopic, endoscopic-assisted, and open resection in 1047 cases. Laryngoscope. 2013; Apr. 123(4):859–69.

24. Lloyd G, Howard D, Phelps P, Cheesman A. Juvenile angiofibroma: the lessons of 20 years of modern imaging. J Laryngol Otol. 1999; Feb. 113(2):127–34.

25. Reyes C, Bentley H, Gelves JA, Solares CA, Byrd JK. Recurrence rate after endoscopic vs. open approaches for juvenile nasopharyngeal angiofibroma: a meta-analysis. J Neurol Surg B Skull Base. 2019; Dec. 80(6):577–85.

26. Huang Y, Liu Z, Wang J, Sun X, Yang L, Wang D. Surgical management of juvenile nasopharyngeal angiofibroma: analysis of 162 cases from 1995 to 2012. Laryngoscope. 2014; Aug. 124(8):1942–6.

27. Liu L, Wang R, Huang D, Han D, Yang W. Multiple factors analysis of intraoperative bleeding and recurrence of juvenile nasopharyngeal angiofibromas. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2001; Jun. 36(3):220–3.
Table 1.
Clinical characteristics of patients with juvenile nasopharyngeal angiofibroma
| Variable | All males (n=78) | No recurrence (n=56) | Recurrence (n=22) | P-value |
|---|---|---|---|---|
| Follow-up period (mo) | 31.4 (7.1–89.6) | 63.1 (24.3–108.7) | 6.6 (6.4–16.6) | <0.01a),* |
| Age at diagnosis (yr) | 0.08b) | |||
| ≤15 | 39 (50.00) | 24 (42.86) | 15 (68.18) | |
| >15 | 39 (50.00) | 32 (57.14) | 7 (31.82) | |
| Year of diagnosis | 0.03b),* | |||
| ≤2010 | 20 (25.64) | 10 (17.86) | 10 (45.45) | |
| >2010 | 58 (75.36) | 46 (82.14) | 12 (54.55) | |
| Tumor size (cm) | <0.01b),* | |||
| ≤4 | 39 (50.00) | 35 (62.50) | 4 (18.18) | |
| >4 | 39 (50.00) | 21 (37.50) | 18 (81.82) | |
| Radkowski stage | 0.01b),* | |||
| I (Ia–Ib) | 24 (30.77) | 21 (37.50) | 3 (13.64) | |
| II (IIa–IIc) | 38 (48.72) | 28 (50.00) | 10 (45.46) | |
| III (IIIa–IIIb) | 16 (20.51) | 7 (12.50) | 9 (40.91) | |
| Sphenoid bone invasion | <0.01b),* | |||
| No | 50 (64.10) | 42 (75.00) | 8 (36.36) | |
| Yes | 28 (35.90) | 14 (25.00) | 14 (63.64) | |
| Preoperative embolization | 0.11b) | |||
| No | 38 (48.72) | 31 (55.36) | 7 (31.82) | |
| Yes | 40 (51.28) | 25 (44.64) | 15 (68.18) | |
| Surgical approach | <0.01b),* | |||
| Endoscopic | 48 (61.54) | 41 (73.21) | 7 (31.82) | |
| Open | 7 (9.00) | 3 (5.36) | 4 (18.18) | |
| Endoscopic and open | 23 (29.49) | 12 (21.43) | 11 (50.00) | |
| Intraoperative bleeding (mL) | <0.01b),* | |||
| <800 | 49 (62.82) | 42 (75.00) | 7 (31.82) | |
| ≥800 | 29 (37.18) | 14 (25.00) | 15 (68.18) |
Table 2.
Univariate and multivariate Cox hazard regression analysis of the risk factors for recurrence of juvenile nasopharyngeal angiofibroma
| Variable |
Univariate analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age at diagnosis (yr) | ||||||
| ≤15 | Ref | Ref | ||||
| >15 | 0.47 | (0.19–1.14) | 0.10 | |||
| Year of diagnosis | ||||||
| Before 2010 | Ref | Ref | Ref | Ref | ||
| After 2010 | 0.40 | (0.17–0.95) | 0.04* | 0.29 | (0.11–0.73) | <0.01* |
| Tumor size (cm) | ||||||
| ≤4 | Ref | Ref | Ref | Ref | ||
| >4 | 5.48 | (1.85–16.25) | <0.01* | 3.12 | (1.00–9.76) | 0.05 |
| Radkowski stage | ||||||
| I (Ia–Ib) | Ref | Ref | ||||
| II (IIa–IIc) | 2.68 | (0.74–9.75) | 0.14 | |||
| III (IIIa–IIIb) | 6.82 | (1.84–25.34) | <0.01* | |||
| Sphenoid bone invasion | ||||||
| No | Ref | Ref | Ref | Ref | ||
| Yes | 4.00 | (1.68–9.57) | <0.01* | 4.10 | (1.60–10.53) | <0.01* |
| Preoperative embolization | ||||||
| No | Ref | Ref | ||||
| Yes | 2.18 | (0.89–5.36) | 0.09 | |||
| Surgical approach | <0.01* | |||||
| Endoscopic | Ref | Ref | Ref | Ref | ||
| Open | 3.75 | (1.09–12.92) | 0.04* | 2.75 | (0.77–9.83) | 0.12 |
| Endoscopic+open | 4.31 | (1.66–11.16) | <0.01* | 4.55 | (1.62–12.75) | <0.01* |
| Intraoperative bleeding (mL) | ||||||
| <800 | Ref | Ref | ||||
| ≥800 | 4.6 | (1.86–11.37) | <0.01* | |||
Table 3.
Cumulative incidence of recurrence among patients with juvenile nasopharyngeal angiofibroma
Table 4.
Frequency of anatomic sites where tumors were residual or recurrent (n=41)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download