Abstract
Objectives.
Our previous study found that multilevel obstructive sleep apnea (OSA) surgery mitigated laryngopharyngeal reflux (LPR) symptoms in terms of the reflux symptom index (RSI), but no studies have investigated the impact of OSA surgery on laryngoscopic parameters. The aim of this study was to examine the clinical outcome of LPR improvement following OSA surgery, with a focus on both the RSI and the reflux finding score (RFS).
Methods.
Prospectively collected data from 28 patients who underwent multilevel OSA surgery from 2017 to 2021 were retrospectively analyzed. Patients were asked to complete the RSI questionnaire and underwent a laryngoscopic examination to evaluate the RFS before and after surgery. Age, height, weight, body mass index (BMI), and polysomnography data before and after surgery were also reviewed.
Results.
After surgery, the total RSI and RFS decreased significantly from 11.96±8.40 to 7.68±6.82 (P=0.003) and from 6.57±3.49 to 3.21±1.87 (P<0.001). The positive rates of RSI and RFS decreased from 28.6% to 17.9% and 32.1% to 0%, respectively. Significant improvements were found in the RSI subdomains of throat clearing, throat mucus, breathing difficulty, troublesome cough, and heartburn sensation, while all RFS subdomains except granuloma improved significantly. In subgroup analyses, no significant differences were found between subgroups based on age, OSA severity, or BMI.
Obstructive sleep apnea (OSA) is a common clinical condition that causes upper airway collapse and airway flow reduction by repeated episodes of apnea and hypopnea during sleep and shows a prevalence of 2%–10% in the adult population [1,2]. OSA is associated with several cardiovascular diseases such as hypertension, arrhythmia, cerebrovascular events, and even neurocognitive impairment and motor vehicle accidents, significantly impacting morbidity and mortality [3-5]. Laryngopharyngeal reflux (LPR) is also a common health problem characterized by acid from the gastrointestinal tract that rises into and damages the upper airway tract mucosa. This condition accounts for approximately 10% of otolaryngology outpatient clinic patients who complain of various bothersome symptoms like chronic cough, a globus sense in the throat, voice change, and dysphagia [6,7]. LPR is diagnosed on the basis of self-reported symptoms, laryngoscopic findings, multi-channel intraluminal impedance, and pH monitoring [8]. However, in clinical settings, the reflux symptom index (RSI) [9] and reflux finding score (RFS) [10], developed by Belafsky, are preferred for their high tolerability, applicability, and low cost.
Several studies have revealed a substantial concurrence between LPR and OSA, which are estimated to co-occur in 30.6%–89.2% of cases, as supported in a recent meta-analysis by Magliulo et al. [11]. Previous studies have suggested an association between these two disease entities based on their common risk factors of obesity, male sex, alcohol intake, and old age [12,13], as well as clinical improvement in LPR following continuous positive airway pressure (CPAP) treatment [14-16]. This improvement further suggests a possible causative relationship between LPR and OSA, even though it remains unclear whether a direct causal link exists [17].
Consistent with our previous study, which found RSI improvement after multilevel OSA surgery [18], another recent study showed LPR symptom relief after OSA surgery [19]. However, these studies have limitations because they did not evaluate clinician-examined laryngoscopic findings and only included self-reported symptom changes based on the RSI questionnaire. The aim of this study was to investigate the therapeutic effect of multilevel OSA surgery on LPR in terms of both symptoms and larynx findings by assessing both RSI and RFS changes following surgery.
We retrospectively reviewed the prospectively collected data of OSA patients who underwent multilevel surgery at a single tertiary medical institution by a single surgeon between September 2017 and April 2021. Multilevel surgery includes uvulopalatopharyngoplasty (UPPP), tonsillectomy, and radiofrequency tongue base reduction, with or without nasal surgery such as septoplasty or turbinoplasty. All patients were asked to complete an RSI questionnaire and underwent endoscopic exam of the larynx before and at least three months after surgery. A total of 86 patients underwent multilevel surgery. Among them, 58 patients were excluded for use of LPR medication or a CPAP before and after surgery and absence of either pre- or post-RSI questionnaires or endoscopic larynx exam. Finally, 28 patients were analyzed for our study. Age, height, weight, body mass index (BMI), and polysomnography (PSG) data before and after surgery were also reviewed. The Institutional Review Board of Samsung Medical Center approved this study (No. 2022-04-009-001; approved on 08 April 2022), and informed consent was waived.
The RSI questionnaire is a widely used, self-administered questionnaire to assess LPR symptom severity. As shown in Table 1, it consists of nine questions regarding subjective symptoms of hoarseness, throat clearing, postnasal drip, swallowing difficulty, coughing, breathing difficulty, troublesome cough, lump sensation, and heartburn over the past month. Each question scale ranges from 0–5, with higher scores indicating more severe symptoms, with a maximum total score of 45. A total score >13 is indicative of LPR [9].
The RFS is a tool to assess LPR severity based on laryngoscopic examination findings. It consists of eight items: subglottic edema, ventricular obliteration, erythema, vocal fold edema, diffuse laryngeal edema, posterior commissure hypertrophy, granuloma, and thick endolaryngeal mucus (Table 2). Higher scores indicate more severe findings, with a maximum total score of 26. A total score >7 is indicative of LPR [10]. Due to the subjective nature of the RFS, there may be discrepancies in scoring between observers. Three otolaryngologists evaluated the scores without perceiving whether they were obtained pre or post operation to ensure the data was as objective as possible. Inter-rater reliability was verified using the kappa value.
To assess the therapeutic effect of OSA surgery on LPR, we compared total pre- and post-operative RSI and RFS. We also compared subdomain scores of pre- and postoperative RSI and RFS to identify factors influenced by OSA surgery. Changes in RSI and RFS were further analyzed according to OSA severity, BMI, age, and surgical outcome. OSA severity was categorized according to the apnea-hypopnea index (AHI) as mild-to-moderate (AHI 5–30) or severe (AHI >30), and surgical outcome was classified as successful if postoperative AHI ≤20 with a 50% reduction from preoperative AHI and unsuccessful if not.
Statistical analysis was performed with IBM SPSS ver. 27.0 (IBM Corp., Armonk, NY, USA). The paired t-test was used to compare RSI and RFS before and after surgery, and the Shapiro-Wilk test was conducted to determine whether the distribution was normal. Wilcoxon signed-rank test was used to compare subdomain scores before and after surgery. Additionally, the Mann-Whitney U-test was used to further compare RSI and RFS changes according to OSA severity, BMI, age, and surgical outcome. Results were considered statistically significant if P<0.05.
Patient characteristics are summarized in Table 3. Among 28 patients, 23 were male and 5 were female. The mean age was 48.4 years (standard deviation [SD], 12.6 years). Their mean BMI was 25.7 kg/m2 (SD, 2.3 kg/m2) with a range of 20.1–29.8 kg/m2. At preoperative PSG, one patient (3.6%) was diagnosed with mild OSA, seven (25.0%) with moderate OSA, and 20 (71.4%) with severe OSA, with a mean AHI score of 39.6/hr (SD, 16.8/hr) and a range of 11.0/hr–62.7/hr. Eight patients (28.6%) had an RSI >13 and nine (32.1%) had an RFS >7.
A comparison of the total RSI and RFS before and after surgery is shown in Fig. 1. Both total RSI and RFS significantly decreased after surgery (by 4.29±6.90, P=0.003 and 3.36±2.63, P<0.001, respectively), reflecting changes from 11.96±8.40 to 7.68±6.82 and 6.57±3.49 to 3.21±1.87, respectively. The RSI positive rate decreased from 28.6% to 17.9%, and the RFS positive rate decreased from 32.1% to 0.0% after surgery. Among the RSI subdomains, throat clearing, throat mucus, breathing difficulty, troublesome cough, and heartburn sensation improved significantly after surgery (Table 4). In the RFS, all subdomains except granuloma improved significantly after surgery (Table 5). The kappa value for the inter-rater reliability of RFS measurements was 0.71.
Tables 6 and 7 show subgroup analyses of changes in RSI and RFS after surgery according to various factors. Regarding RSI changes, the subgroups with mild to moderate OSA, BMI ≥25 kg/m2, age ≥50 years, and unsuccessful surgical outcomes showed greater improvements (8.50 vs. 2.60, 5.11 vs. 2.56, 5.13 vs. 3.31, and 7.20 vs. 1.92, respectively), although a statistically significant difference was only found for surgical outcomes (P=0.049). Regarding RFS changes, the subgroups with severe OSA, BMI ≥25 kg/m2, age <50 years, and unsuccessful surgical outcomes showed more improvement (3.35 vs. 3.13, 3.79 vs. 2.22, 3.62 vs. 3.00, and 3.70 vs. 2.85, respectively), but no statistically significant differences were observed (P=0.862, 0.142, 0.586, and 0.313, respectively).
Due to the high co-occurrence of LPR in OSA patients [11-13,20,21], many clinicians have investigated clinical improvements in LPR after OSA management such as CPAP or surgery [14-16, 18,19,22]. Those studies confirmed LPR improvement after treatment, but only in terms of self-reported symptoms, except for our previous study reporting both RSI and RFS improvement after CPAP treatment [16]. In contrast, in this study, we demonstrated the clinical efficacy of OSA surgery on LPR based on both symptom relief and changes in laryngoscopic findings. Both laryngeal reflux symptoms and laryngoscopic findings significantly improved after OSA surgery, demonstrating more advanced results than our previous study that only evaluated the post-surgical RSI response [18].
Although several studies have revealed a relationship between LPR and OSA, the exact mechanism and direct correlation are still unclear. Several authors have suggested that increased negative intrathoracic pressure generated by increased respiratory effort during apnea-hypopnea events induces relaxation of the lower esophageal sphincter and repeated arousal, and shallow sleep caused by apnea-hypopnea events could increase susceptibility to gastroesophageal reflux (GER) in OSA patients, but no causal relationship has been clearly shown [23]. Rather, the role of the upper esophageal sphincter that protects the upper airway mucosa is thought to be more important in LPR than in GER. One crucial pathophysiological condition is lower esophageal sphincter dysfunction, which is considered a distinct disease entity and not solely a manifestation of GER [24,25]. Eventually, esophageal sphincter dysfunction results in laryngeal mucosal inflammation and laryngeal reflux symptoms even with minimal esophageal reflux. Such repetitive inflammation could also induce exacerbation of OSA by direct narrowing of the upper airway due to inflammation-mediated tissue hypertrophy and by the impairment of reflexes in the upper airway tract induced by sensory dysfunction. These result in an upper airway that is vulnerable to collapse, eventually becoming part of a vicious cycle [17,26].
Herein, we pose several hypotheses for why LPR is relieved after multilevel OSA surgery. First, OSA surgery could directly reduce the mechanical trauma that could be induced during snoring and airway collapse by resecting and correcting the flexible structure of the laryngopharyngeal airway. It can also reduce respiratory effort, lower intrathoracic pressure, and protect against GER. Second, by improving mouth breathing, one of the main clinical manifestations of OSA, the humidification function of the upper airway mucosa is preserved to promote laryngeal tissue healing and mucosal homeostasis. An animal study showed that repetitive pressure changes and collapse of the upper airway tract could induce inflammatory changes that can lead to tissue damage [27].
In our study, the RSI and RFS positive rates decreased after surgery from 28.6% to 17.9% and 32.1% to 0%, respectively. Additionally, most subdomains of the RSI and RFS significantly improved after surgery. Throat clearing, throat mucus, breathing difficulty, troublesome cough, and heartburn sensation showed significant improvements among the RSI domains, while all subdomains in the RFS except granuloma, which was not observed in any patient at any time point, improved significantly after surgery. We offer several hypotheses to explain the higher negative conversion rate of the RFS and the significant improvements in each subdomain compared with the RSI. Because the patients included in this study mainly complained of snoring and apnea, not LPR itself, patients with mild LPR might have been preferentially included in our study, and it is highly likely that some people with severe LPR were excluded because they received other treatment. Therefore, even in patients with positive baseline RSI and RFS, the highest observed values were not very close to the maximum possible score, and most patients scored just above the cut-off value, as shown in Fig. 1B. Therefore, even only slight improvements could have led to a relatively high conversion rate, especially for RFS responses because the maximum preoperative RFS was only 13, corresponding to just half of the perfect score. Furthermore, postoperative discomfort such as swallowing difficulty and globus sensation after OSA surgery could be confounding factors because those complaints have similarities to several subdomains in the RSI, which is a self-reported scoring system. These factors may explain why the conversion rate of the RSI was not as high as that of the RFS. Lastly, the small number of patients in this study might have contributed to this result.
We also analyzed whether there were differences in therapeutic effects according to OSA severity, BMI, age, and surgical outcomes measured by PSG. Each subgroup showed improvements in the RSI and RFS after surgery, but no changes were statistically significant except for changes in the RSI in the subgroup analysis according to surgical outcomes. Some explanations can be proposed for these findings. First, because not all patients underwent follow-up PSG, this subgroup analysis included only 23 patients after excluding five patients. It is possible that these five patients reported good surgical outcomes regarding OSA symptoms and therefore refused a follow-up test. Additionally, many patients who underwent OSA surgery at our center tended to have early loss to follow-up, before the 3-month postoperative point, which is also presumed to have been due to dramatic symptom improvement after surgery. This is unlike CPAP treatment, which requires regular follow-up, but it nevertheless contributed to the small sample size in this study and limited the representation of real-world phenomena. Second, in terms of RSI changes according to surgical outcomes, we should take into account that the preoperative RSI values for the unsuccessful group were about 1.5 times greater than those in the successful group (15.70 vs. 10.38), which could account for these differences. However, both groups showed the almost same postoperative RSI (about 8.5), implying that LPR symptom alleviation could be achieved regardless of the OSA outcome determined by PSG values. Additionally, unlike the RFS, because the RSI is a self-reporting questionnaire, postoperative discomfort could act as a confounding factor because it could not be clearly distinguished from most RSI subdomains. Third, the use of PSG and AHI as the sole surgical outcome measures could be debated, and reproducibility was not addressed as we conducted only one follow-up PSG examination. An interesting finding is that the changes in both the RFS and RSI were as much as twice as large in more obese patients (BMI ≥25 kg/m2: 3.79 and 5.11, respectively) than in less obese patients (BMI <25 kg/m2: 2.22 and 2.56, respectively), although the differences were not statistically significant. The more obese patients had higher average preoperative RSI and RFS (12.95 and 6.74, respectively) than the less obese patients (9.89 and 6.00, respectively), which also implies a stronger positive correlation between OSA and LPR in obese patients than non-obese patients, as reported in a previous study [28].
Despite promising results and advances beyond previous studies about the clinical effect of OSA treatment on LPR, this study has several limitations. First, this was a retrospective study without a control group, and it was not conducted in a blinded fashion. Second, our results could have been weakened by patient selection bias in each surgical modality, the small number of patients that were treated at a single institution and who were operated on by a single surgeon, and a short follow-up period. Third, differences in anatomical positions and the severity of anatomical obstacles by position could affect LPR severity. Furthermore, surgical outcomes in terms of LPR improvement might have been affected by the combination of surgical methods. While all 28 patients received UPPP, 22 underwent tongue base reduction surgery, 16 underwent septoplasty, and three underwent partial epiglottectomy, all of which were performed as part of multilevel surgery. Therefore, large and comprehensive studies regarding whether and how these anatomical factors influence LPR and surgical outcomes are needed. Fourth, even though we added the RFS as a more reliable and objective method, we did not include more objective tests like multichannel intraluminal impedance and pH monitoring. Novel techniques detecting LPR have recently been developed, such as the pepsin salivary test [29] and airway pH monitoring using an oropharyngeal probe [30]. Hence, further studies with a longer follow-up period and large cohort studies with more objective tools are required to validate our results.
In conclusion, for OSA management, multilevel surgery has potential therapeutic effects on the LPR in terms of both laryngeal reflux symptoms and laryngoscopic examination findings. Future studies with more objective tools are required to establish the clinical impact of OSA surgery on LRP in greater depth.
▪ Multilevel surgery for obstructive sleep apnea (OSA) also offers potential therapeutic effects on laryngopharyngeal reflux (LPR) in terms of both symptom mitigation and laryngoscopic examination findings.
▪ After multilevel OSA surgery, statistically significant differences were found in the total reflux symptom index (RSI) and reflux finding score (RFS), as well as the majority of their subdomains of RSI and RFS.
▪ The severity of OSA, body mass index, age, and surgical outcomes did not appear to be clearly related to the effect on LPR.
ACKNOWLEDGMENTS
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MTIE) (20016285).
REFERENCES
1. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993; Apr. 328(17):1230–5.

2. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015; Apr. 3(4):310–8.

3. Harding SM. Complications and consequences of obstructive sleep apnea. Curr Opin Pulm Med. 2000; Nov. 6(6):485–9.

4. Jean-Louis G, Zizi F, Clark LT, Brown CD, McFarlane SI. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med. 2008; Jun. 4(3):261–72.

5. Garbarino S, Durando P, Guglielmi O, Dini G, Bersi F, Fornarino S, et al. Sleep apnea, sleep debt and daytime sleepiness are independently associated with road accidents: a cross-sectional study on truck drivers. PLoS One. 2016; Nov. 11(11):e0166262.

6. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991; Apr. 101(4 Pt 2 Suppl 53):1–78.

7. Jaspersen D, Kulig M, Labenz J, Leodolter A, Lind T, Meyer-Sabellek W, et al. Prevalence of extra-oesophageal manifestations in gastrooesophageal reflux disease: an analysis based on the ProGERD study. Aliment Pharmacol Ther. 2003; Jun. 17(12):1515–20.

8. Lechien JR, Akst LM, Hamdan AL, Schindler A, Karkos PD, Barillari MR, et al. Evaluation and management of laryngopharyngeal reflux disease: state of the art review. Otolaryngol Head Neck Surg. 2019; May. 160(5):762–82.

9. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002; Jun. 16(2):274–7.

10. Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope. 2001; Aug. 111(8):1313–7.

11. Magliulo G, Iannella G, Polimeni A, De Vincentiis M, Meccariello G, Gulotta G, et al. Laryngopharyngeal reflux in obstructive sleep apnoea patients: literature review and meta-analysis. Am J Otolaryngol. 2018; Nov-Dec. 39(6):776–80.

12. Wise SK, Wise JC, DelGaudio JM. Gastroesophageal reflux and laryngopharyngeal reflux in patients with sleep-disordered breathing. Otolaryngol Head Neck Surg. 2006; Aug. 135(2):253–7.

13. Elhennawi DM, Ahmed MR, Abou-Halawa AS. Correlation of obstructive sleep apnoea and laryngopharyngeal reflux: phmetry study. Clin Otolaryngol. 2016; Dec. 41(6):758–61.

14. Sundar KM, Daly SE, Willis AM. A longitudinal study of CPAP therapy for patients with chronic cough and obstructive sleep apnoea. Cough. 2013; Jul. 9(1):19.

15. Wang L, Han H, Wang G, Liu H, Sun Z, Li B, et al. Relationship between reflux diseases and obstructive sleep apnea together with continuous positive airway pressure treatment efficiency analysis. Sleep Med. 2020; Nov. 75:151–5.

16. Choi JH, Lee E, Hong SD, Chung SK, Jung YG, Kim HY. Potential therapeutic effect of continuous positive airway pressure on laryngopharyngeal reflux in obstructive sleep apnea patients. J Clin Med. 2021; Jun. 10(13):2861.

17. Eskiizmir G, Kezirian E. Is there a vicious cycle between obstructive sleep apnea and laryngopharyngeal reflux disease. Med Hypotheses. 2009; Nov. 73(5):706–8.

18. Kim SJ, Kim HY, Jeong JI, Hong SD, Chung SK, Dhong HJ. Changes in the reflux symptom index after multilevel surgery for obstructive sleep apnea. Clin Exp Otorhinolaryngol. 2017; Sep. 10(3):259–64.

19. Yue R, Xing D, Qin J, Lu H, Liu C, Li S, et al. The effect of obstructive sleep apnea surgery on laryngopharyngeal reflux with obstructive sleep apnea. Acta Otolaryngol. 2020; Aug. 140(8):697–701.

20. Caparroz F, Campanholo M, Stefanini R, Vidigal T, Haddad L, Bittencourt LR, et al. Laryngopharyngeal reflux and dysphagia in patients with obstructive sleep apnea: is there an association. Sleep Breath. 2019; Jun. 23(2):619–26.

21. Gouveia CJ, Yalamanchili A, Ghadersohi S, Price CP, Bove M, Attarian HP, et al. Are chronic cough and laryngopharyngeal reflux more common in obstructive sleep apnea patients. Laryngoscope. 2019; May. 129(5):1244–9.

22. Tawk M, Goodrich S, Kinasewitz G, Orr W. The effect of 1 week of continuous positive airway pressure treatment in obstructive sleep apnea patients with concomitant gastroesophageal reflux. Chest. 2006; Oct. 130(4):1003–8.

23. Shepherd K, Hillman D, Holloway R, Eastwood P. Mechanisms of nocturnal gastroesophageal reflux events in obstructive sleep apnea. Sleep Breath. 2011; Sep. 15(3):561–70.

24. Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. Ear Nose Throat J. 2002; Sep. 81(9 Suppl 2):7–9.
25. Payne RJ, Kost KM, Frenkiel S, Zeitouni AG, Sejean G, Sweet RC, et al. Laryngeal inflammation assessed using the reflux finding score in obstructive sleep apnea. Otolaryngol Head Neck Surg. 2006; May. 134(5):836–42.

26. Nguyen AT, Jobin V, Payne R, Beauregard J, Naor N, Kimoff RJ. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep. 2005; May. 28(5):585–93.

27. Almendros I, Carreras A, Ramirez J, Montserrat JM, Navajas D, Farre R. Upper airway collapse and reopening induce inflammation in a sleep apnoea model. Eur Respir J. 2008; Aug. 32(2):399–404.

28. Rodrigues MM, Dibbern RS, Santos VJ, Passeri LA. Influence of obesity on the correlation between laryngopharyngeal reflux and obstructive sleep apnea. Braz J Otorhinolaryngol. 2014; Jan-Feb. 80(1):5–10.

Fig. 1.
Comparison of the total reflux symptom index (RSI) and reflux finding score (RFS) before and after obstructive sleep apnea surgery. (A) Statistically significant differences in both the total RSI and RFS. (B) Individual total RSI (B1) and RFS (B2) values before and after surgery. Values are presented as mean±standard deviation.
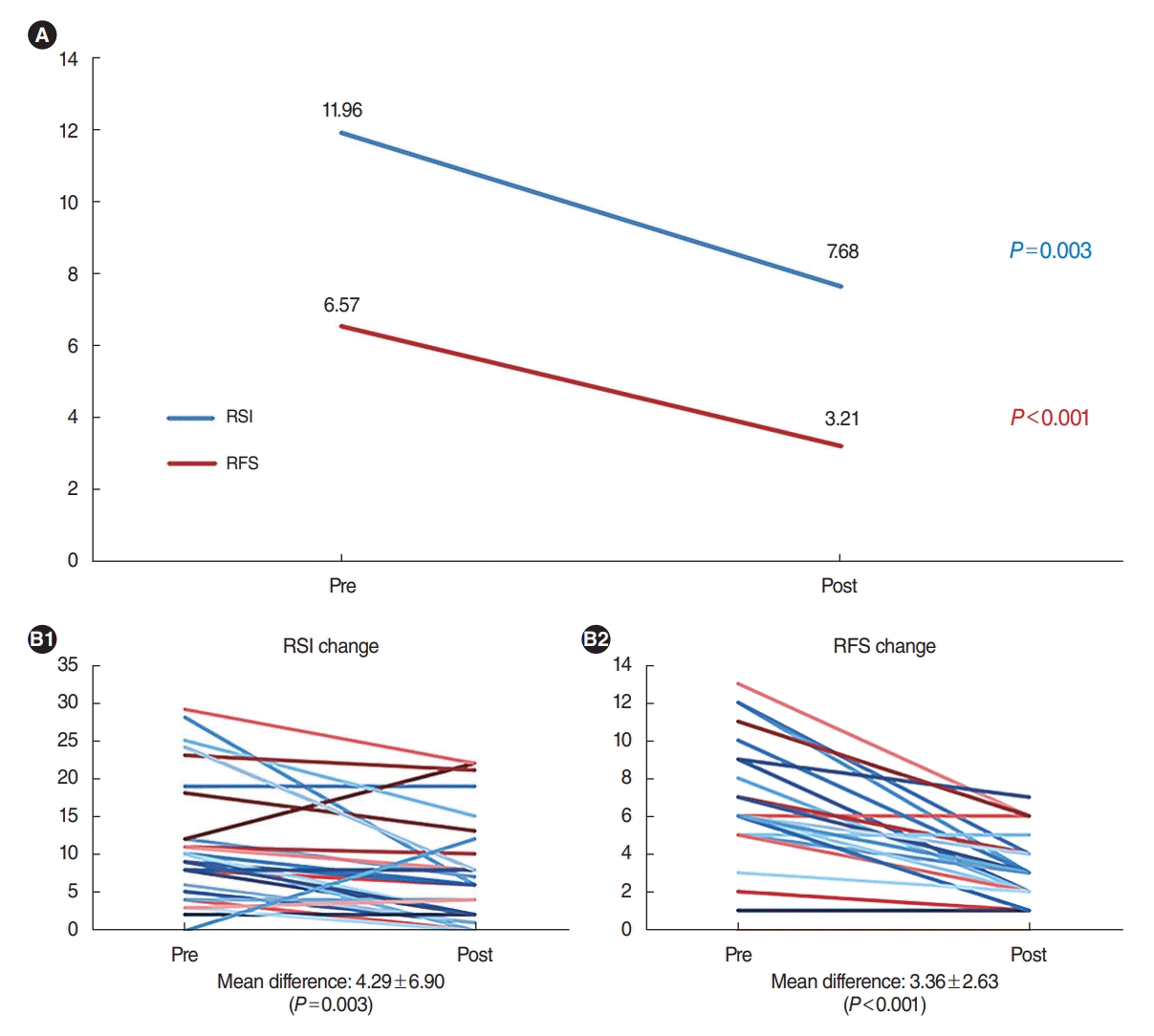
Table 1.
Reflux symptom index questionnaire
| Within the last month, how did the following problem affect you? | Scorea) | |||||
|---|---|---|---|---|---|---|
| Hoarseness or a problem with your voice | 0 | 1 | 2 | 3 | 4 | 5 |
| Clearing your throat | 0 | 1 | 2 | 3 | 4 | 5 |
| Excess throat mucus or postnasal drip | 0 | 1 | 2 | 3 | 4 | 5 |
| Difficulty swallowing food, liquids, or pills | 0 | 1 | 2 | 3 | 4 | 5 |
| Coughing after you ate or after lying down | 0 | 1 | 2 | 3 | 4 | 5 |
| Breathing difficulties or choking episodes | 0 | 1 | 2 | 3 | 4 | 5 |
| Troublesome or annoying cough | 0 | 1 | 2 | 3 | 4 | 5 |
| Sensation of something sticking in your throat or a lump in your throat | 0 | 1 | 2 | 3 | 4 | 5 |
| Heartburn, chest pain, indigestion, or stomach acid coming up | 0 | 1 | 2 | 3 | 4 | 5 |
| Total | ||||||
Table 2.
Calculation of the reflux finding score
Table 3.
Summary of patient characteristics
Table 4.
Comparison of total RSI and subdomains before and after OSA surgery
| Variable | Before surgery | After surgery | P-valuea) |
|---|---|---|---|
| Hoarseness | 1.04±1.26 | 0.68±0.98 | 0.059 |
| Throat clearing | 1.79±1.10 | 1.25±1.27 | 0.032 |
| Throat mucus | 2.32±1.54 | 1.50±1.14 | 0.006 |
| Dysphagia | 0.54±1.14 | 0.79±1.23 | 0.265 |
| Coughing | 1.00±1.52 | 0.57±0.74 | 0.138 |
| Breathing difficulty | 1.25±1.46 | 0.54±0.74 | 0.019 |
| Troublesome cough | 1.07±1.46 | 0.50±0.75 | 0.029 |
| Foreign body sensation | 1.57±1.50 | 1.71±1.41 | 0.670 |
| Heartburn sensation | 1.39±1.74 | 0.79±1.13 | 0.002 |
| Total | 11.96±8.40 | 7.68±6.82 | 0.003 |
Table 5.
Comparison of total RFS and subdomains before and after OSA surgery
| Variable | Before surgery | After surgery | P-valuea) |
|---|---|---|---|
| Subglottic edema | 0.36±0.78 | 0.07±0.38 | 0.046 |
| Ventricular obliteration | 1.00±1.02 | 0.43±0.84 | 0.005 |
| Erythema | 1.86±1.53 | 0.86±1.01 | <0.001 |
| Vocal fold edema | 0.64±0.73 | 0.25±0.52 | 0.005 |
| Diffuse laryngeal edema | 0.75±0.75 | 0.29±0.46 | 0.001 |
| Posterior commissure hypertrophy | 1.04±0.92 | 0.75±0.75 | 0.021 |
| Granuloma | 0.0±0.0 | 0.0±0.0 | NA |
| Thick laryngeal mucus | 0.93±1.02 | 0.57±0.92 | 0.025 |
| Total | 6.57±3.49 | 3.21±1.87 | <0.001 |
Table 6.
Subgroup analysis of the total RSI before and after OSA surgery
| Variable | Before surgery | After surgery | Difference | P-valuea) |
|---|---|---|---|---|
| OSA severity | 0.123 | |||
| Mild-to-moderate (n=8) | 16.63±9.97 | 8.13±7.38 | 8.50±5.71 | |
| Severe (n=20) | 10.10±7.14 | 7.50±6.78 | 2.60±6.72 | |
| BMI (kg/m2) | 0.383 | |||
| <25 (n=9) | 9.89±7.45 | 7.33±3.91 | 2.56±7.25 | |
| ≥25 (n=19) | 12.95±8.83 | 7.84±7.93 | 5.11±6.77 | |
| Age (yr) | 0.555 | |||
| <50 (n=13) | 9.46±6.67 | 6.15±5.55 | 3.31±6.32 | |
| ≥50 (n=15) | 14.13±9.34 | 9.00±7.70 | 5.13±7.47 | |
| Surgical outcome | 0.049 | |||
| Successful (n=13) | 10.38±8.37 | 8.46±7.02 | 1.92±5.04 | |
| Unsuccessful (n=10) | 15.70±9.32 | 8.50±7.59 | 7.20±9.10 |
Table 7.
Subgroup analysis of total RFS before and after OSA surgery
| Variable | Before surgery | After surgery | Difference | P-valuea) |
|---|---|---|---|---|
| OSA severity | 0.862 | |||
| Mild-to-moderate (n=8) | 6.13±3.40 | 3.00±1.85 | 3.13±2.64 | |
| Severe (n=20) | 6.65±3.50 | 3.30±1.92 | 3.35±2.70 | |
| BMI (kg/m2) | 0.142 | |||
| <25 (n=9) | 6.00±3.74 | 3.78±2.33 | 2.22±2.11 | |
| ≥25 (n=19) | 6.74±3.33 | 2.95±1.61 | 3.79±2.72 | |
| Age (yr) | 0.586 | |||
| <50 (n=13) | 6.77±3.35 | 3.15±1.58 | 3.62±2.72 | |
| ≥50 (n=15) | 6.27±3.58 | 3.27±2.15 | 3.00±2.56 | |
| Surgical outcome | 0.313 | |||
| Successful (n=13) | 6.08±4.27 | 3.23±2.17 | 2.85±2.97 | |
| Unsuccessful (n=10) | 7.30±2.50 | 3.60±1.78 | 3.70±2.21 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download