INTRODUCTION
Actinomycosis most commonly occurs in the head and neck region, and it rarely develops in the paranasal sinus (PNS) [
1]. Fungal ball (FB) is the most common category of noninvasive fungal rhinosinusitis and the most common form of unilateral chronic rhinosinusitis [
2]. To our knowledge, actinomycosis and FB rarely coexist in the PNS with only three reported English cases [
3-
5]. The clinical manifestations and radiologic findings of actinomycosis and FB are similar [
1,
2,
4,
6], so it is difficult to differentiate actinomycosis from FB without a histopathological examination. Although actinomycosis and FB are treated similarly, long-term antibiotic therapy is indicated in actinomycosis [
1,
6]. However, shortterm antibiotic therapy for six weeks was recently recommended [
4,
7]. We report a case of coexisting actinomycosis and FB that was successfully treated via endoscopic sinus surgery (ESS) and short-term antibiotic therapy. A review of the relevant literature was also conducted.
Go to :

CASE REPORT
A 65-year-old man was referred to our clinic for the evaluation and management of right rhinosinusitis, incidentally found on magnetic resonance imaging (MRI), and performed to evaluate his complaint of dizziness. He had no history of nasal trauma, nasal surgery, or odontogenic procedures. He reported a medical history of hypertension and diabetes mellitus. The patient exhibited no rhinologic symptoms. Endoscopic examination revealed a nasal septum deviation to the right as well as a grey and brown lesion with mucopurulent discharge in the right middle meatus. T1-weighted MRI showed an isointense signal in the maxillary sinus, while T2-weighted MRI revealed a hypointense signal (
Fig. 1). The computed tomography (CT) scan revealed a soft tissue and calcific densities in the maxillary sinus and middle meatus (
Fig. 2). Based on these findings, the patient was provisionally diagnosed with FB. ESS was performed under local anesthesia. Intraoperatively, a hard grey and brown lesion as well as unhealthy inflamed mucosa was observed in the maxillary sinus and anterior ethmoid sinus (
Fig. 3). Histopathologic examination revealed septate hyphae with 45° branches on hematoxylin and eosin (H&E) staining (
Fig. 4A). The septate hyphae were highlighted on Gomori methenamine silver (GMS) staining (
Fig. 4B). These findings were consistent with
Aspergillus. Sulfur granules were observed on H&E staining (
Fig. 4A). Moreover, the GMS staining showed thin filamentous organisms in the sulfur granules (
Fig. 4B), confirming the diagnosis of actinomycosis. Histopathologic examination of the specimen confirmed the diagnosis of actinomycosis accompanied by FB. Ceftriaxone was intravenously administered for three days postoperatively. He received oral cefpodoxime for four weeks. During the 2-year follow-up period, the patient exhibited no signs of recurrence (
Fig. 5).
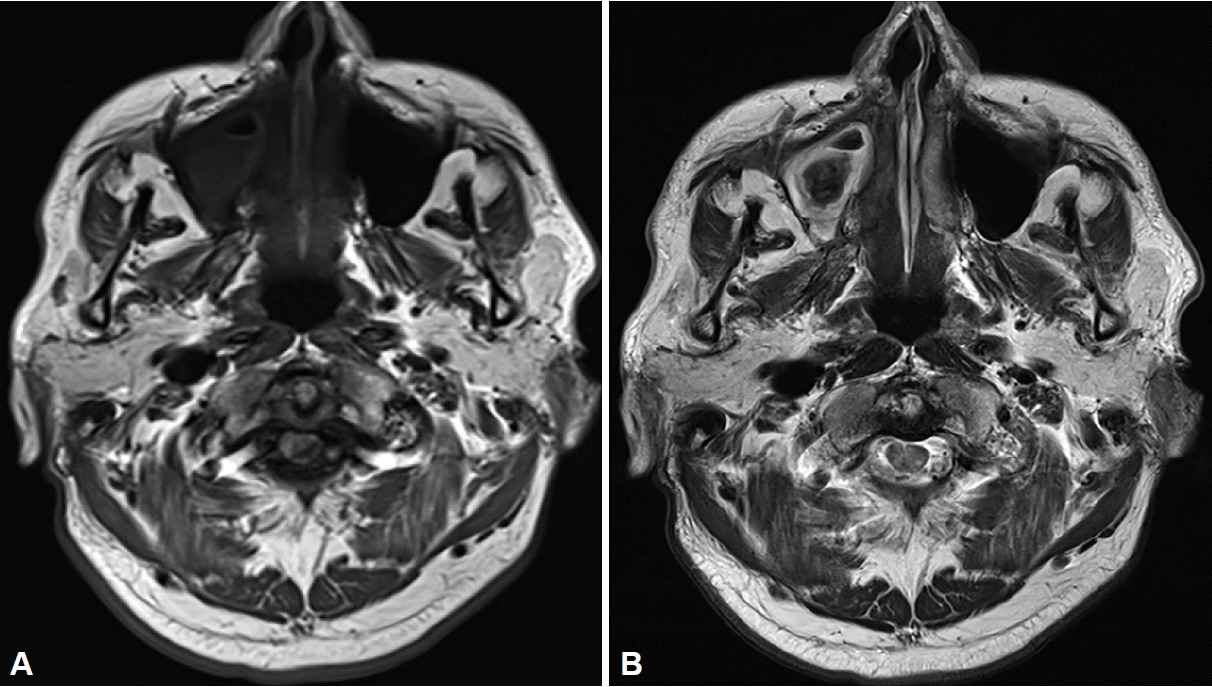 | Fig. 1.Magnetic resonance imaging (MRI). A: T1-weighted MRI displaying isointense signal in the right maxillary sinus. B: T2-weighted MRI demonstrating a hypointense signal, called signal voiding, in the right maxillary sinus. 
|
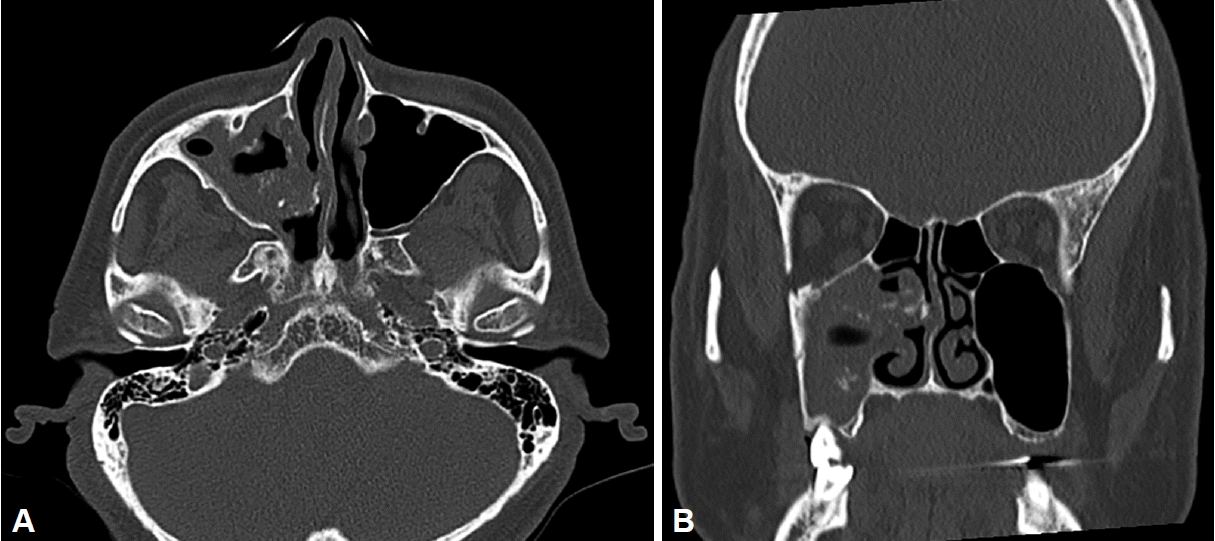 | Fig. 2.Computed tomography scan showing partial opacification, with calcification, in the right maxillary sinus (A: axial, B: coronal). 
|
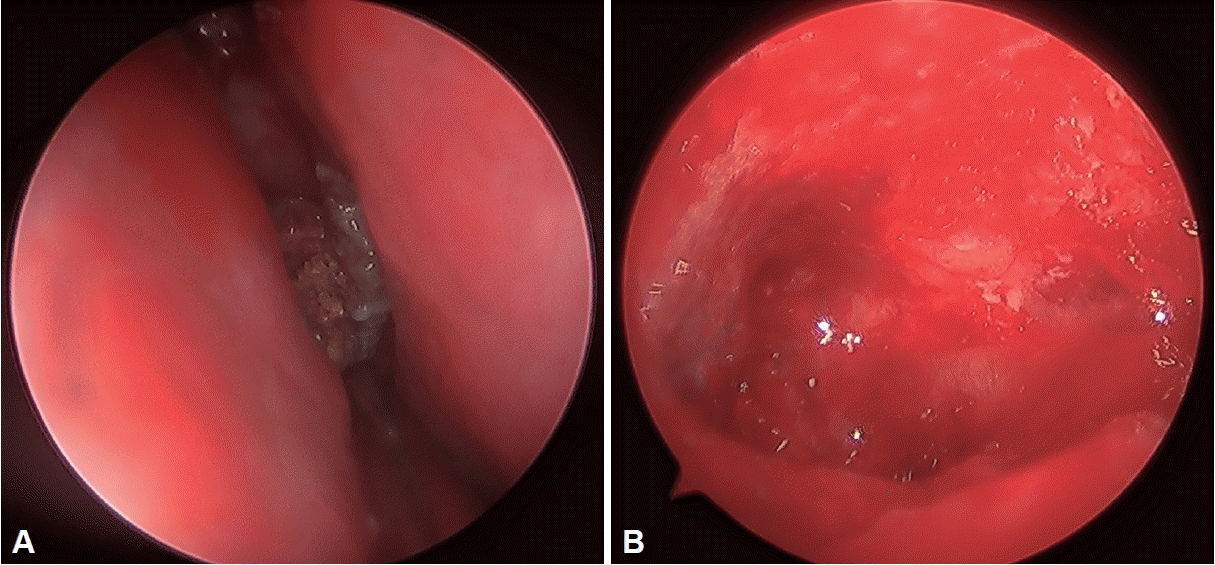 | Fig. 3.Intraoperative findings. A: The hard grey and brown lesion is observed in the right middle meatus. B: The severely inflamed mucosa is found in the right maxillary sinus. 
|
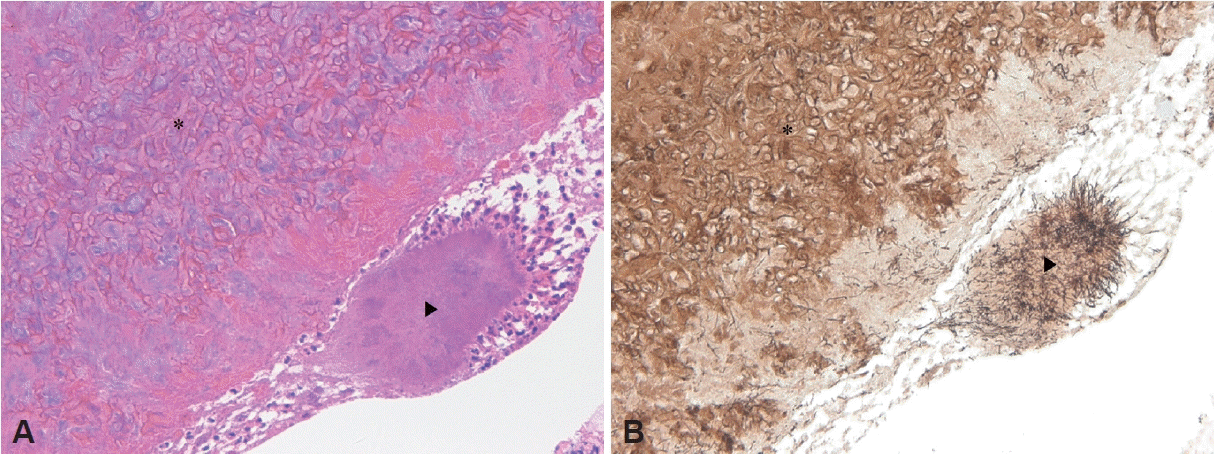 | Fig. 4.Histopathologic findings. A: Hematoxylin and Eosin staining (×200) showing a sulfur granule surrounded by neutrophilic inflammatory cells (arrowhead) and fungal hyphae (asterisk). B: Gomori methenamine silver statin (×200) revealing thin filamentous rods in the sulfur granule of actinomycosis (arrowhead) and septae branching at a 45° (asterisk), consistent with Aspergillus. 
|
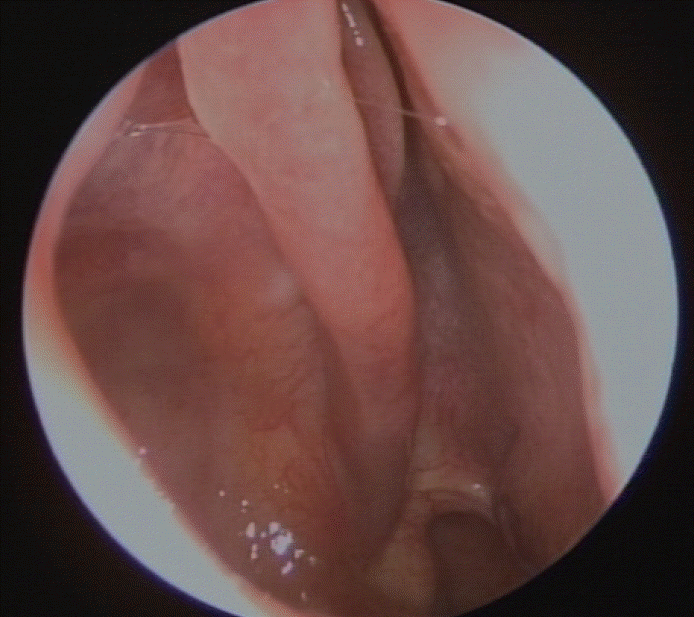 | Fig. 5.Postoperatively endoscopic finding showing the healthy mucosa in the right middle meatus. No evidence of recurrence is
seen. 
|
Go to :

DISCUSSION
Actinomycosis is caused by
Actinomyces, a gram-positive, anaerobic bacterium, typically found in the oral cavity and aerodigestive tract [
1,
6,
7]. FB is a type of extramucosal fungal rhinosinusitis caused by
Aspergillus spp. [
8]. FB is the most common form of unilateral chronic rhinosinusitis [
2], but Actinomycosis of the PNS is rare [
1,
6]. Coexisting actinomycosis and FB, as seen in this case, rarely occurs in the PNS. Although the pathogeneses of FB and actinomycosis remain unclear, they share some similarities. Its pathogenesis is reportedly associated with mucosal injury, caused by dental procedures or ostial obstruction, resulting in sinus hypoxia [
1,
4-
6,
8]. Of two theories, it is thought that ostial obstruction may be more likely than mucosal injury because of having no history of dental treatment or facial trauma in our case. Therefore, it is inferred that ostial obstruction, due to nasal mucosa swelling or inflammation, could lead to the anaerobic state in which the growth of
Aspergillus and
Actinomyces may be facilitated.
The presenting symptoms and endoscopic findings of actinomycosis and FB were similar to those associated with chronic rhinosinusitis [
1,
2,
6,
8]. Based on three previously reported cases of coexisting actinomycosis and FB, the clinical and endoscopic findings were suggestive of chronic rhinosinusitis. However, in one case, a hard greyish-black mass was found on endoscopic examination [
3-
5]. On CT scans, actinomycosis is characterized by opacities in the involved sinus, bony sclerosis of the sinus wall, and calcifications, which are similar to the findings of FB [
1,
2,
4,
6,
8]. The MRI findings of actinomycosis were reportedly non-specific. Meanwhile, FB is characterized by signal voiding, which may be mistaken for air [
8]. In our case, the patient reported no rhinologic symptoms, but grey and brown-colored material was observed on the endoscopic examination. Calcification and partial opacification were observed on the CT scan results, whereas signal voiding was appreciated on T2-weighted MRI. Consequently, the patient was provisionally diagnosed with FB.
Actinomycosis is diagnosed based on the culture findings or histopathological examination [
1]. Its diagnosis is typically confirmed via histopathologic examination because actinobacteria are difficult to detect in culture studies since they are anaerobic. FB is also diagnosed via histopathologic examination [
8]. The histopathological findings of actinomycosis include sulfur granules, defined as basophilic masses with eosinophilic terminal clubs and filamentous gram-positive rods, on H&E staining. Meanwhile, FB is characterized by narrow septate hyphae and 45° dichotomous branching hyphae. Special staining techniques, such as GMS and periodic acid-Schiff, are useful for diagnosing actinomycosis and FB. The characteristic findings of actinomycosis and FB were observed in our case. Thus, the patient was diagnosed with coexisting actinomycosis and FB.
The treatment of choice for actinomycosis and FB is surgical excision. Among the surgical techniques, ESS was preferred because it facilitated total excision and restoration of ventilation [
1,
2,
4-
6,
8]. Although additional therapy is not required in FB [
2,
8], medical therapy, specifically antibiotic administration following surgical excision, is recommended for actinomycosis [
1,
4-
7].
Actinomyces are susceptible to various antimicrobial agents, including penicillin, doxycycline, macrolides, clindamycin, piperacillin/tazobactam, imipenem, and cephalosporins. The duration of antibiotic therapy varies from six weeks to 12 months [
1,
5,
6]. Traditionally, long-term antibiotic therapy has been done but the application of short-term antibiotic therapy (within six weeks) after surgery has been recently reported [
4,
7]. In our case, the patient underwent ESS and short-term antibiotic therapy for four weeks, resulting in a satisfactory result without recurrence and complications. Short-term antibiotic therapy following ESS was sufficient to treat actinomycosis. This regimen was also applicable for cases of coexisting actinomycosis and FB.
In summary, coexistent actinomycosis and FB are extremely rare. It is difficult to distinguish actinomycosis from FB because their clinical, endoscopic, and CT findings are similar. A missed diagnosis of actinomycosis results in failure to prevent the disease from worsening. Therefore, a high index of suspicion is important to accurately diagnose patients with actinomycosis and FB. Moreover, a thorough microscopic examination is critical. Finally, surgical removal of actinomycosis and FB as well as short-term antibiotic therapy were effective to prevent recurrence and aggressive progression of actinomycosis.
Go to :









 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download