Abstract
Background
Methods
Results
Supplementary Information
Notes
Ethics Statement
All procedures performed in the current study were approved by the Wayne State University IRB and/or national research ethics committee (063419M1X; 08/13/2019) in accordance with the 1964 Helsinki declaration and its later amendments. Formal written informed consent was not required with a waiver by the appropriate IRB and/or national research ethics committee.
Availability of Data and Material
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Author contributions
Conceptualization: EA, RAF. Data curation: EA, HJ, SK. Formal analysis: HJ, SK. Investigation: EA, RAF, SB. Methodology: EA, RAF, SB, SK. Project administration: EA, RAF. Resources: EA, RAF. Supervision: RAF. Visualization: EA, RAF, SB. Writing—original draft preparation: EA. Writing—review and editing: EA, HJ, SK, RAF, SB.
References
Fig. 1.
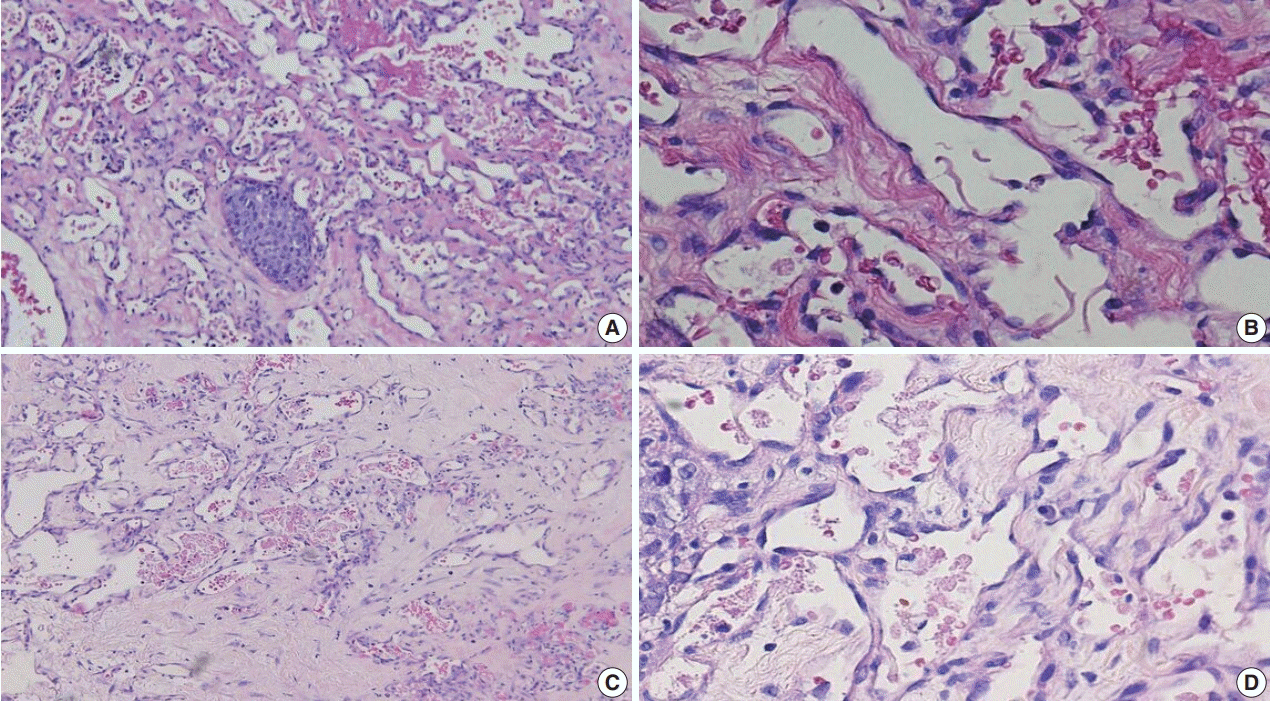
Fig. 2.
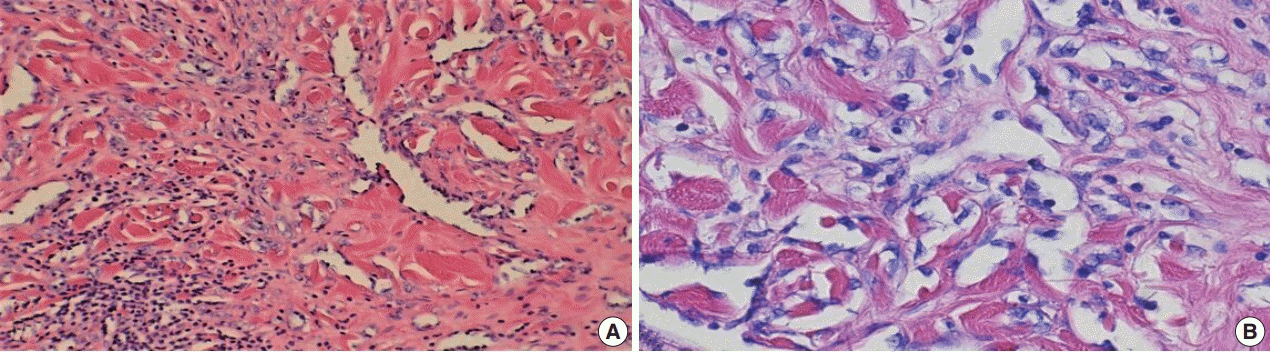
Fig. 3.
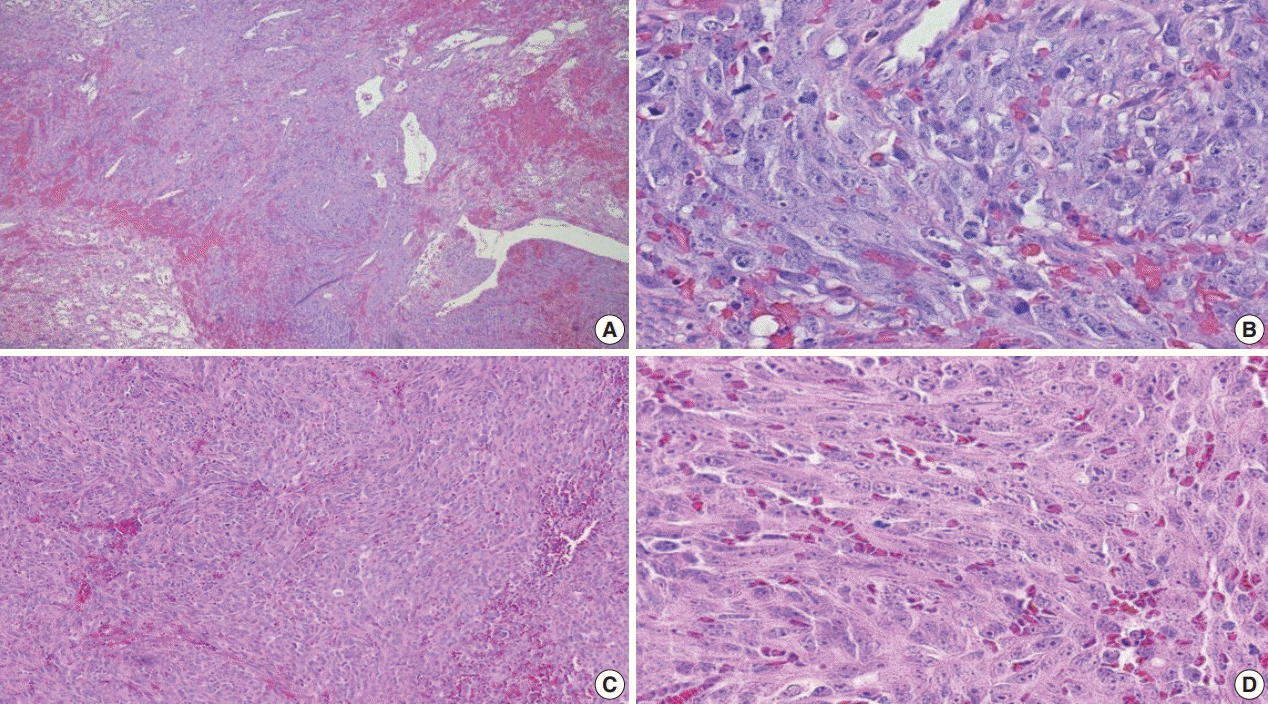
Fig. 4.
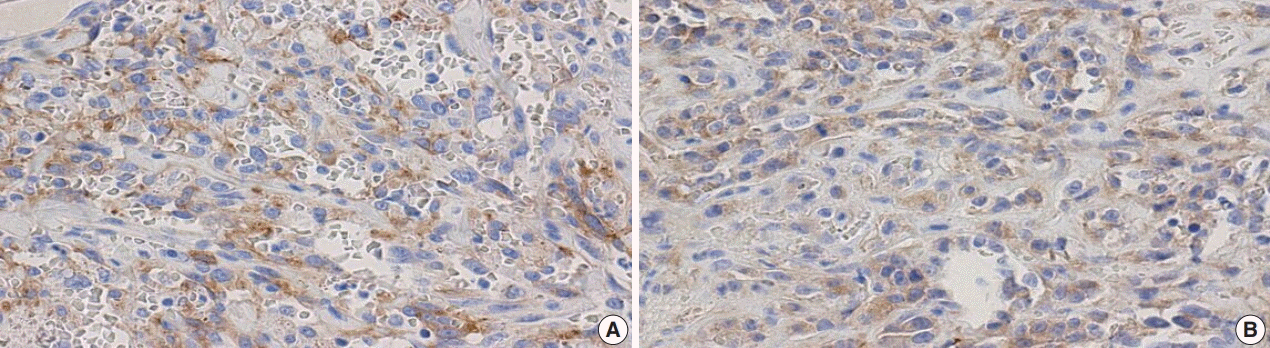
Fig. 6.
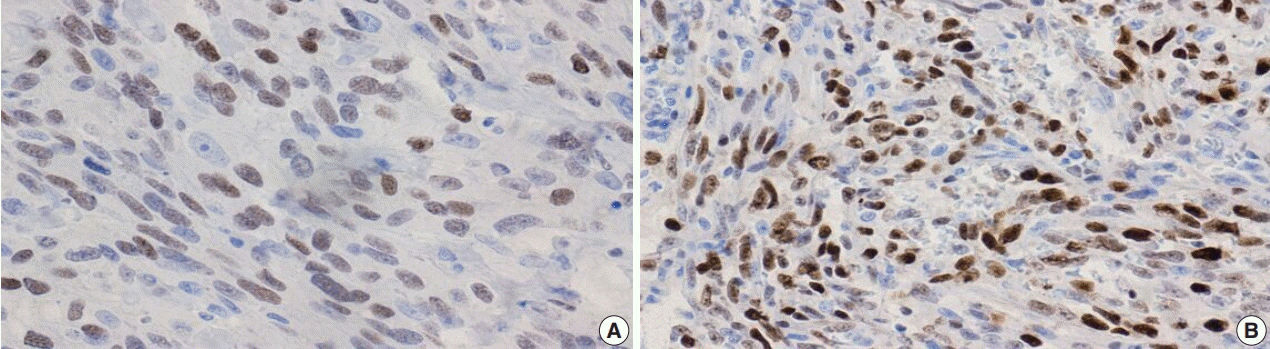
Fig. 7.
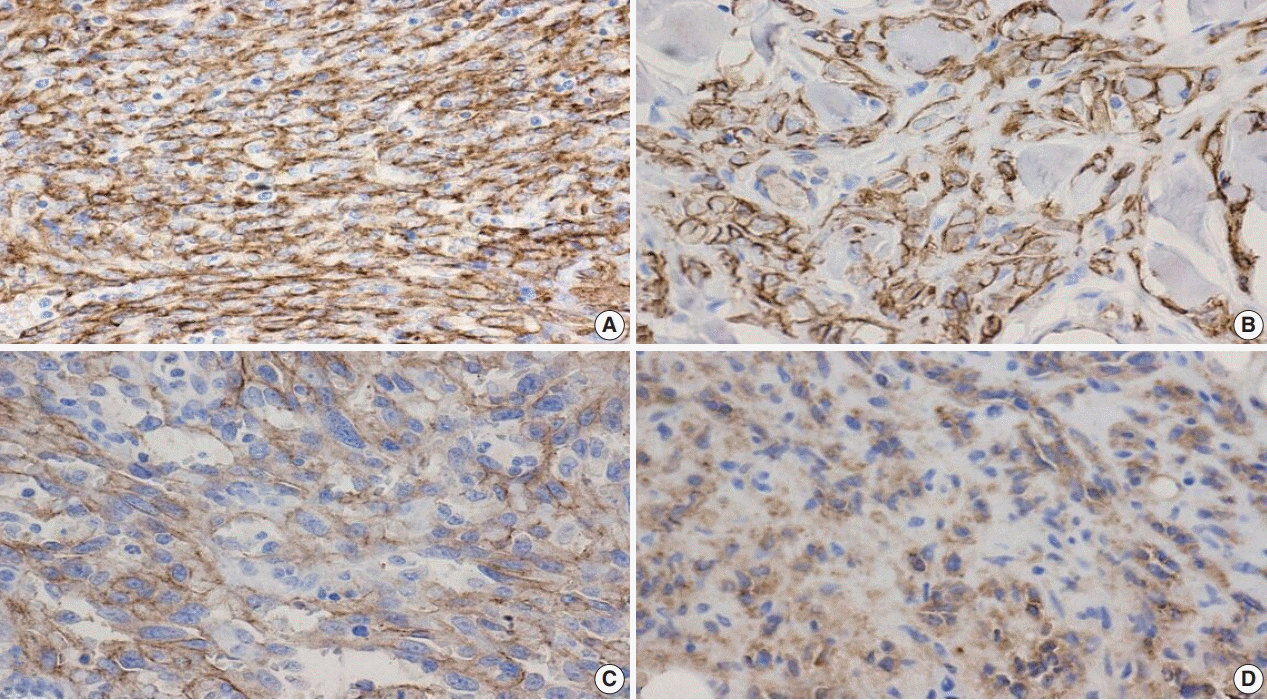
Fig. 8.
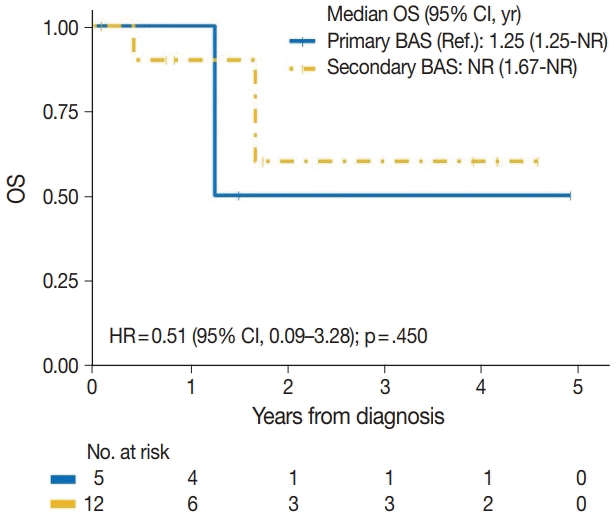
Fig. 9.
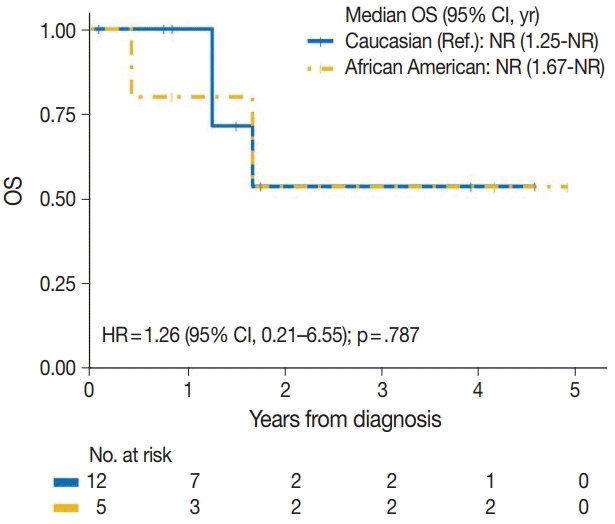
Fig. 10.
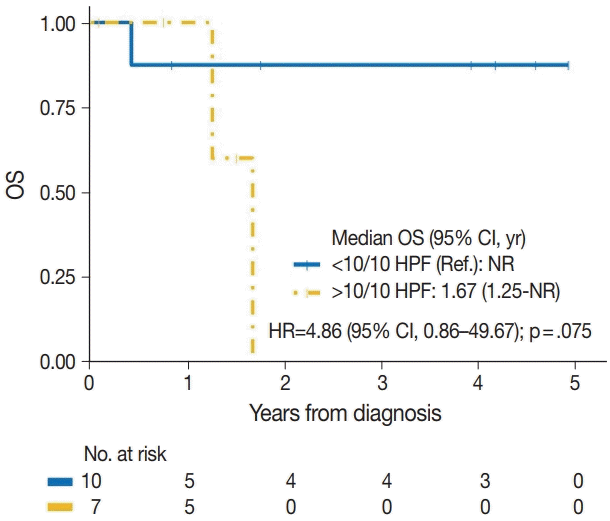
Fig. 11.
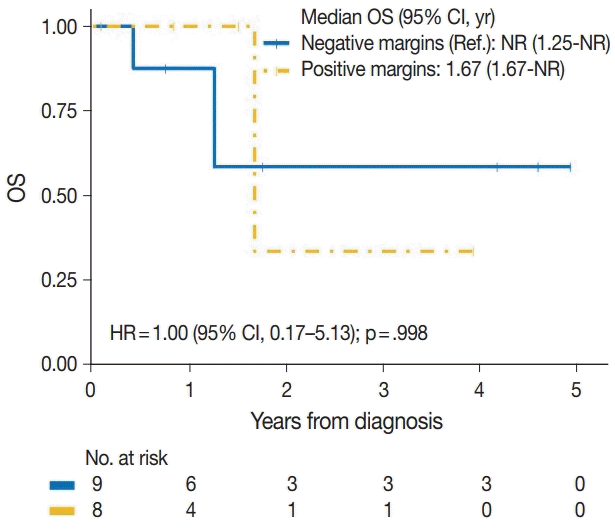
Fig. 12.
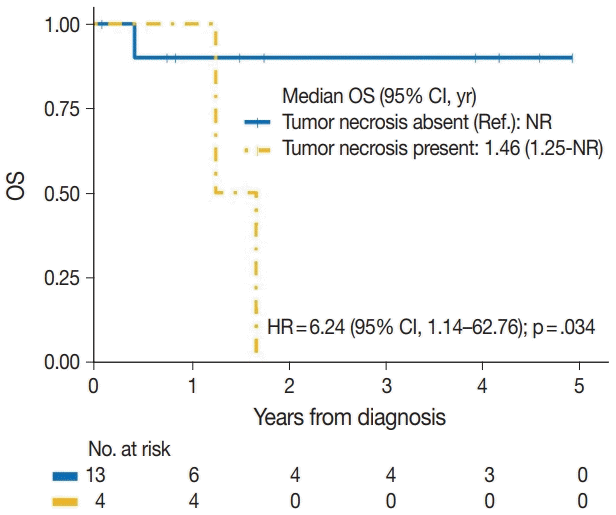
Fig. 13.
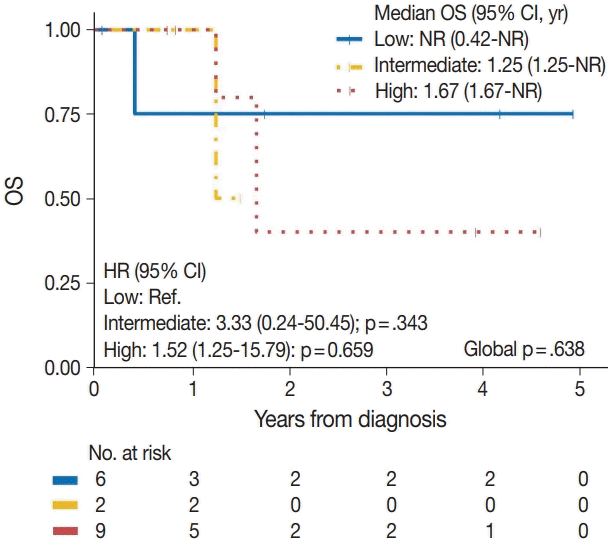
Fig. 14.
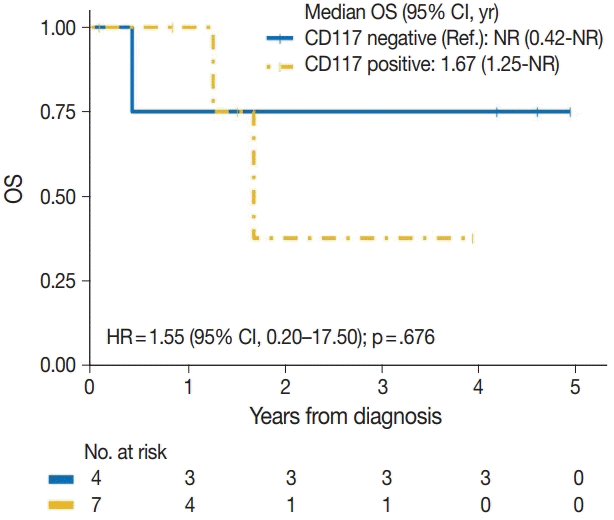
Fig. 15.
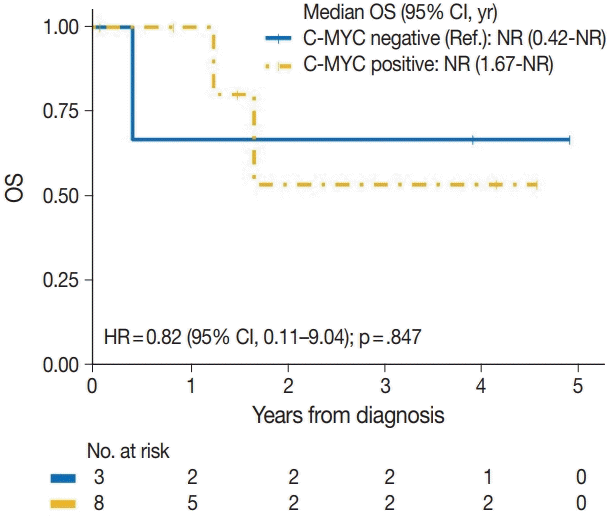
Table 1.
Table 2.
| Variable | Total (n = 11) | Primary BAS (n = 3) | Secondary BAS (n = 8) | p-valuea |
|---|---|---|---|---|
| CD117 | > .99 | |||
| Positive | 7 (63.6) | 2 (66.7) | 5 (62.5) | |
| Negative | 4 (36.4) | 1 (33.3) | 3 (37.5) | |
| p53 | .236 | |||
| Positive | 4 (36.4) | 0 | 4 (50.0) | |
| Negative | 7 (63.6) | 3 (100) | 4 (50.0) | |
| D-240 | > .99 | |||
| Positive | 7 (63.6) | 2 (66.7) | 5 (62.5) | |
| Negative | 4 (36.4) | 1 (33.3) | 3 (37.5) | |
| CD31 | > .99 | |||
| Positive | 11 (100) | 3 (100) | 8 (100) | |
| Negative | 0 | 0 | 0 | |
| C-MYC | > .99 | |||
| Positive | 8 (72.7) | 2 (66.7) | 6 (75.0) | |
| Negative | 3 (27.3) | 1 (33.3) | 2 (25.0) |
Table 3.
| Variable | Total (n = 17) | Primary BAS (n = 5) | Secondary BAS (n = 12) | p-valuea |
|---|---|---|---|---|
| Age at diagnosis (yr) | 66 (23–76) | 36 (23–71) | 67 (33–76) | .246 |
| Race | > .99 | |||
| Caucasian | 12 (70.6) | 4 (80.0) | 8 (66.7) | |
| African American | 5 (29.4) | 1 (20.0) | 4 (33.3) | |
| Tumor size (cm) | 1.1 (0.4–28) | 2 (0.5–28) | 0.95 (0.4–2.5) | .437 |
| Missing | 8 | 2 | 6 | |
| Tumor grade | .087 | |||
| Low | 6 (35.3) | 2 (40.0) | 4 (33.3) | |
| Intermediate | 2 (11.8) | 2 (40.0) | 0 | |
| High | 9 (52.9) | 1 (20.0) | 8 (66.7) | |
| Tumor necrosis | .538 | |||
| Yes | 4 (23.5) | 2 (40.0) | 2 (16.7) | |
| No | 13 (76.5) | 3 (60.0) | 10 (83.3) | |
| Mitotic count | .593 | |||
| > 10/10 HPF | 7 (41.2) | 3 (60.0) | 4 (33.3) | |
| < 10/10 HPF | 10 (58.8) | 2 (40.0) | 8 (66.7) | |
| Lymph node metastasis | .191 | |||
| Yes | 3 (17.6) | 2 (40.0) | 1 (8.3) | |
| No | 14 (82.4) | 3 (60.0) | 11 (91.7) | |
| Positive margins | > .99 | |||
| Yes | 8 (47.1) | 2 (40.0) | 6 (50.0) | |
| No | 9 (52.9) | 3 (60.0) | 6 (50.0) | |
| Tumor site | .294 | |||
| Right | 9 (52.9) | 4 (80.0) | 5 (41.7) | |
| Left | 8 (47.1) | 1 (20.0) | 7 (58.3) | |
| Multifocal tumors | > .99 | |||
| Yes | 4 (23.5) | 1 (20.0) | 3 (25.0) | |
| No | 13 (76.5) | 4 (80.0) | 9 (75.0) | |
| Skin involved | .044 | |||
| Yes | 7 (41.2) | 0 | 7 (58.3) | |
| No | 10 (58.8) | 5 (100) | 5 (41.7) | |
| Obesity | .117 | |||
| Yes | 12 (70.6) | 2 (40.0) | 10 (83.3) | |
| No | 5 (29.4) | 3 (60.0) | 2 (16.7) | |
| Hypertension | .102 | |||
| Yes | 6 (35.3) | 0 | 6 (50.0) | |
| No | 11 (64.7) | 5 (100) | 6 (50.0) | |
| Smoking | > .99 | |||
| Yes | 4 (23.5) | 1 (20.0) | 3 (25.0) | |
| No | 13 (76.5) | 4 (80.0) | 9 (75.0) | |
| Diabetes | .338 | |||
| Yes | 7 (41.2) | 1 (20.0) | 6 (50.0) | |
| No | 10 (58.8) | 4 (80.0) | 6 (50.0) | |
| Treatment received | .353 | |||
| Surgery only | 14 (82.4) | 4 (80.0) | 10 (83.3) | |
| Surgery + chemotherapy | 2 (11.8) | 0 | 2 (16.7) | |
| Surgery + radiation + chemotherapy | 1 (5.9) | 1 (20.0) | 0 |
Table 4.
| Variable | Event/No. | HR (95% CI) | p-value |
|---|---|---|---|
| Age at diagnosis | 5/17 | 1.01 (0.96–1.07) | .845 |
| Race | |||
| Caucasian | 3/12 | Reference | |
| African American | 2/5 | 1.26 (0.21–6.55) | .787 |
| BAS type | |||
| Primary BAS | 2/5 | Reference | |
| Secondary BAS | 3/12 | 0.51 (0.09–3.28) | .450 |
| Tumor size (cm) | 3/9 | 1.04 (0.95–1.13) | .307 |
| Tumor grade | .638a | ||
| Low | 1/6 | Reference | |
| Intermediate | 1/2 | 3.33 (0.24–50.45) | .343 |
| High | 3/9 | 1.52 (0.25–15.79) | .659 |
| Tumor necrosis | |||
| No | 1/13 | Reference | |
| Yes | 4/4 | 6.24 (1.14–62.76) | .034 |
| Mitotic count | |||
| < 10/10 HPF | 1/10 | Reference | |
| > 10/10 HPF | 4/7 | 4.86 (0.86–49.67) | .075 |
| Lymph node metastasis | |||
| No | 3/14 | Reference | |
| Yes | 2/3 | 2.61 (0.42–14.00) | .278 |
| Positive margins | |||
| No | 3/9 | Reference | |
| Yes | 2/8 | 1.00 (0.17–5.13) | .998 |
| Tumor site | |||
| Left | 2/8 | Reference | |
| Right | 3/9 | 1.35 (0.26–8.26) | .719 |
| Multifocal tumors | |||
| No | 5/13 | Reference | |
| Yes | 0/4 | 0.37 (0.003–3.32) | .439 |
| Skin involved | |||
| No | 3/10 | Reference | |
| Yes | 2/7 | 0.78 (0.13–4.14) | .773 |
| Obesity | |||
| No | 3/5 | Reference | |
| Yes | 2/12 | 0.20 (0.03–1.10) | .063 |
| Hypertension | |||
| No | 4/11 | Reference | |
| Yes | 1/6 | 0.99 (0.10–5.40) | .990 |
| Smoking | |||
| No | 5/13 | Reference | |
| Yes | 0/4 | 0.45 (0.003–4.04) | .551 |
| Diabetes | |||
| No | 4/10 | Reference | |
| Yes | 1/7 | 0.58 (0.06–3.17) | .548 |
| Treatment received | |||
| Surgery only | 4/14 | Reference | |
| Surgery + chemotherapy/radiotherapy | 1/3 | 1.52 (0.15–8.29) | .671 |
| CD117 | |||
| Negative | 1/4 | Reference | |
| Positive | 2/7 | 1.55 (0.20–17.50) | .676 |
| p53 | |||
| Negative | 2/7 | Reference | |
| Positive | 1/4 | 1.23 (0.11–9.37) | .847 |
| D2-40 | |||
| Negative | 1/4 | Reference | |
| Positive | 2/7 | 0.95 (0.12–10.49) | .960 |
| c-Myc | |||
| Negative | 1/3 | Reference | |
| Positive | 2/8 | 0.82 (0.11–9.04) | .847 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download