1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64:73–84.

2. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014; 384:766–81.
3. Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010; 5:145–71.

4. Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017; 65:1132–44.

5. Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020; 152:116–41.

6. Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss-Isakov N, Hahn M, Webb M, Shibolet O, et al. Serum malondialdehyde is associated with non-alcoholic fatty liver and related liver damage differentially in men and women. Antioxidants (Basel). 2020; 9:578.

7. Sekiya M, Hiraishi A, Touyama M, Sakamoto K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem Biophys Res Commun. 2008; 375:602–7.

8. Ferramosca A, Di Giacomo M, Zara V. Antioxidant dietary approach in treatment of fatty liver: new insights and updates. World J Gastroenterol. 2017; 23:4146–57.

9. Germain K, Kim PK. Pexophagy: a model for selective autophagy. Int J Mol Sci. 2020; 21:578.

10. Sugiura A, Mattie S, Prudent J, McBride HM. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived preperoxisomes. Nature. 2017; 542:251–4.

11. Smith JJ, Aitchison JD. Peroxisomes take shape. Nat Rev Mol Cell Biol. 2013; 14:803–17.
12. Lodhi IJ, Semenkovich CF. Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab. 2014; 19:380–92.

13. Kovacs WJ, Charles KN, Walter KM, Shackelford JE, Wikander TM, Richards MJ, et al. Peroxisome deficiency-induced ER stress and SREBP-2 pathway activation in the liver of newborn Pex2 knock-out mice. Biochim Biophys Acta. 2012; 1821:895–907.

14. Li X, Baumgart E, Morrell JC, Jimenez-Sanchez G, Valle D, Gould SJ. PEX11 beta deficiency is lethal and impairs neuronal migration but does not abrogate peroxisome function. Mol Cell Biol. 2002; 22:4358–65.

15. Hwang I, Lee J, Huh JY, Park J, Lee HB, Ho YS, et al. Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes. 2012; 61:728–38.

16. Hwang I, Uddin MJ, Pak ES, Kang H, Jin EJ, Jo S, et al. The impaired redox balance in peroxisomes of catalase knockout mice accelerates nonalcoholic fatty liver disease through endoplasmic reticulum stress. Free Radic Biol Med. 2020; 148:22–32.

17. Piao L, Dorotea D, Jiang S, Koh EH, Oh GT, Ha H. Impaired peroxisomal fitness in obese mice, a vicious cycle exacerbating adipocyte dysfunction via oxidative stress. Antioxid Redox Signal. 2019; 31:1339–51.
18. Islam S, Won J, Khan M, Chavin KD, Singh I. Peroxisomal footprint in the pathogenesis of nonalcoholic steatohepatitis. Ann Hepatol. 2020; 19:466–71.

19. Farnier M. Update on the clinical utility of fenofibrate in mixed dyslipidemias: mechanisms of action and rational prescribing. Vasc Health Risk Manag. 2008; 4:991–1000.

20. Montagner A, Polizzi A, Fouche E, Ducheix S, Lippi Y, Lasserre F, et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016; 65:1202–14.

21. Jo SH, Nam H, Lee J, Park S, Lee J, Kyoung DS. Fenofibrate use is associated with lower mortality and fewer cardiovascular events in patients with diabetes: results of 10,114 patients from the Korean National Health Insurance Service Cohort. Diabetes Care. 2021; 44:1868–76.

22. Elam MB, Ginsberg HN, Lovato LC, Corson M, Largay J, Leiter LA, et al. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017; 2:370–80.

23. Sohn M, Kim K, Uddin MJ, Lee G, Hwang I, Kang H, et al. Delayed treatment with fenofibrate protects against high-fat dietinduced kidney injury in mice: the possible role of AMPK autophagy. Am J Physiol Renal Physiol. 2017; 312:F323–34.

24. Weng H, Ji X, Endo K, Iwai N. Pex11a deficiency is associated with a reduced abundance of functional peroxisomes and aggravated renal interstitial lesions. Hypertension. 2014; 64:1054–60.

25. Lee JN, Dutta RK, Kim SG, Lim JY, Kim SJ, Choe SK, et al. Fenofibrate, a peroxisome proliferator-activated receptor α ligand, prevents abnormal liver function induced by a fastingrefeeding process. Biochem Biophys Res Commun. 2013; 442:22–7.

26. Araki H, Tamada Y, Imoto S, Dunmore B, Sanders D, Humphrey S, et al. Analysis of PPARalpha-dependent and PPARalpha-independent transcript regulation following fenofibrate treatment of human endothelial cells. Angiogenesis. 2009; 12:221–9.

27. Hua H, Yang J, Lin H, Xi Y, Dai M, Xu G, et al. PPARα-independent action against metabolic syndrome development by fibrates is mediated by inhibition of STAT3 signalling. J Pharm Pharmacol. 2018; 70:1630–42.

28. Akiyama TE, Nicol CJ, Fievet C, Staels B, Ward JM, Auwerx J, et al. Peroxisome proliferator-activated receptor-alpha regulates lipid homeostasis, but is not associated with obesity: studies with congenic mouse lines. J Biol Chem. 2001; 276:39088–93.
29. Kondo K, Sugioka T, Tsukada K, Aizawa M, Takizawa M, Shimizu K, et al. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, improves hepatic microcirculatory patency and oxygen availability in a high-fat-diet-induced fatty liver in mice. Adv Exp Med Biol. 2010; 662:77–82.
30. Kostapanos MS, Kei A, Elisaf MS. Current role of fenofibrate in the prevention and management of non-alcoholic fatty liver disease. World J Hepatol. 2013; 5:470–8.

31. Walton PA, Brees C, Lismont C, Apanasets O, Fransen M. The peroxisomal import receptor PEX5 functions as a stress sensor, retaining catalase in the cytosol in times of oxidative stress. Biochim Biophys Acta Mol Cell Res. 2017; 1864:1833–43.

32. Imanaka T, Aihara K, Suzuki Y, Yokota S, Osumi T. The 70- kDa peroxisomal membrane protein (PMP70), an ATP-binding cassette transporter. Cell Biochem Biophys. 2000; 32:131–8.
33. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015; 62:720–33.

34. Regnier M, Polizzi A, Smati S, Lukowicz C, Fougerat A, Lippi Y, et al. Hepatocyte-specific deletion of Pparα promotes NAFLD in the context of obesity. Sci Rep. 2020; 10:6489.

35. Jain MR, Giri SR, Bhoi B, Trivedi C, Rath A, Rathod R, et al. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 2018; 38:1084–94.

36. Rakhshandehroo M, Knoch B, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010; 2010:612089.

37. Cheng S, Liang S, Liu Q, Deng Z, Zhang Y, Du J, et al. Diosgenin prevents high-fat diet-induced rat non-alcoholic fatty liver disease through the AMPK and LXR signaling pathways. Int J Mol Med. 2018; 41:1089–95.

38. Chen WL, Chen YL, Chiang YM, Wang SG, Lee HM. Fenofibrate lowers lipid accumulation in myotubes by modulating the PPARα/AMPK/FoxO1/ATGL pathway. Biochem Pharmacol. 2012; 84:522–31.

39. Yan F, Wang Q, Xu C, Cao M, Zhou X, Wang T, et al. Peroxisome proliferator-activated receptor α activation induces hepatic steatosis, suggesting an adverse effect. PLoS One. 2014; 9:e99245.

40. Oosterveer MH, Grefhorst A, van Dijk TH, Havinga R, Staels B, Kuipers F, et al. Fenofibrate simultaneously induces hepatic fatty acid oxidation, synthesis, and elongation in mice. J Biol Chem. 2009; 284:34036–44.

41. Cortes-Lopez F, Sanchez-Mendoza A, Centurion D, CervantesPerez LG, Castrejon-Tellez V, Del Valle-Mondragon L, et al. Fenofibrate protects cardiomyocytes from hypoxia/reperfusion- and high glucose-induced detrimental effects. PPAR Res. 2021; 2021:8895376.

42. Ning LJ, He AY, Lu DL, Li JM, Qiao F, Li DL, et al. Nutritional background changes the hypolipidemic effects of fenofibrate in Nile tilapia (Oreochromis niloticus). Sci Rep. 2017; 7:41706.

43. Honsho M, Tamura S, Shimozawa N, Suzuki Y, Kondo N, Fujiki Y. Mutation in PEX16 is causal in the peroxisome-deficient Zellweger syndrome of complementation group D. Am J Hum Genet. 1998; 63:1622–30.
44. Sacksteder KA, Jones JM, South ST, Li X, Liu Y, Gould SJ. PEX19 binds multiple peroxisomal membrane proteins, is predominantly cytoplasmic, and is required for peroxisome membrane synthesis. J Cell Biol. 2000; 148:931–44.

45. Ghaedi K, Tamura S, Okumoto K, Matsuzono Y, Fujiki Y. The peroxin pex3p initiates membrane assembly in peroxisome biogenesis. Mol Biol Cell. 2000; 11:2085–102.

46. Delille HK, Dodt G, Schrader M. Pex11pβ-mediated maturation of peroxisomes. Commun Integr Biol. 2011; 4:51–4.

47. Weng H, Ji X, Naito Y, Endo K, Ma X, Takahashi R, et al. Pex11α deficiency impairs peroxisome elongation and division and contributes to nonalcoholic fatty liver in mice. Am J Physiol Endocrinol Metab. 2013; 304:E187–96.

48. Walker CL, Pomatto L, Tripathi DN, Davies K. Redox regulation of homeostasis and proteostasis in peroxisomes. Physiol Rev. 2018; 98:89–115.

49. Piao L, Choi J, Kwon G, Ha H. Endogenous catalase delays high-fat diet-induced liver injury in mice. Korean J Physiol Pharmacol. 2017; 21:317–25.

50. Shin SK, Cho HW, Song SE, Bae JH, Im SS, Hwang I, et al. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflugers Arch. 2019; 471:829–43.

51. Koepke JI, Wood CS, Terlecky LJ, Walton PA, Terlecky SR. Progeric effects of catalase inactivation in human cells. Toxicol Appl Pharmacol. 2008; 232:99–108.

52. Hwang I, Uddin MJ, Lee G, Jiang S, Pak ES, Ha H. Peroxiredoxin 3 deficiency accelerates chronic kidney injury in mice through interactions between macrophages and tubular epithelial cells. Free Radic Biol Med. 2019; 131:162–72.

53. Wang YX. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. 2010; 20:124–37.

54. Schrader M, Grille S, Fahimi HD, Islinger M. Peroxisome interactions and cross-talk with other subcellular compartments in animal cells. Subcell Biochem. 2013; 69:1–22.

55. Li X, Gould SJ. PEX11 promotes peroxisome division independently of peroxisome metabolism. J Cell Biol. 2002; 156:643–51.

56. Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017; 16:203.

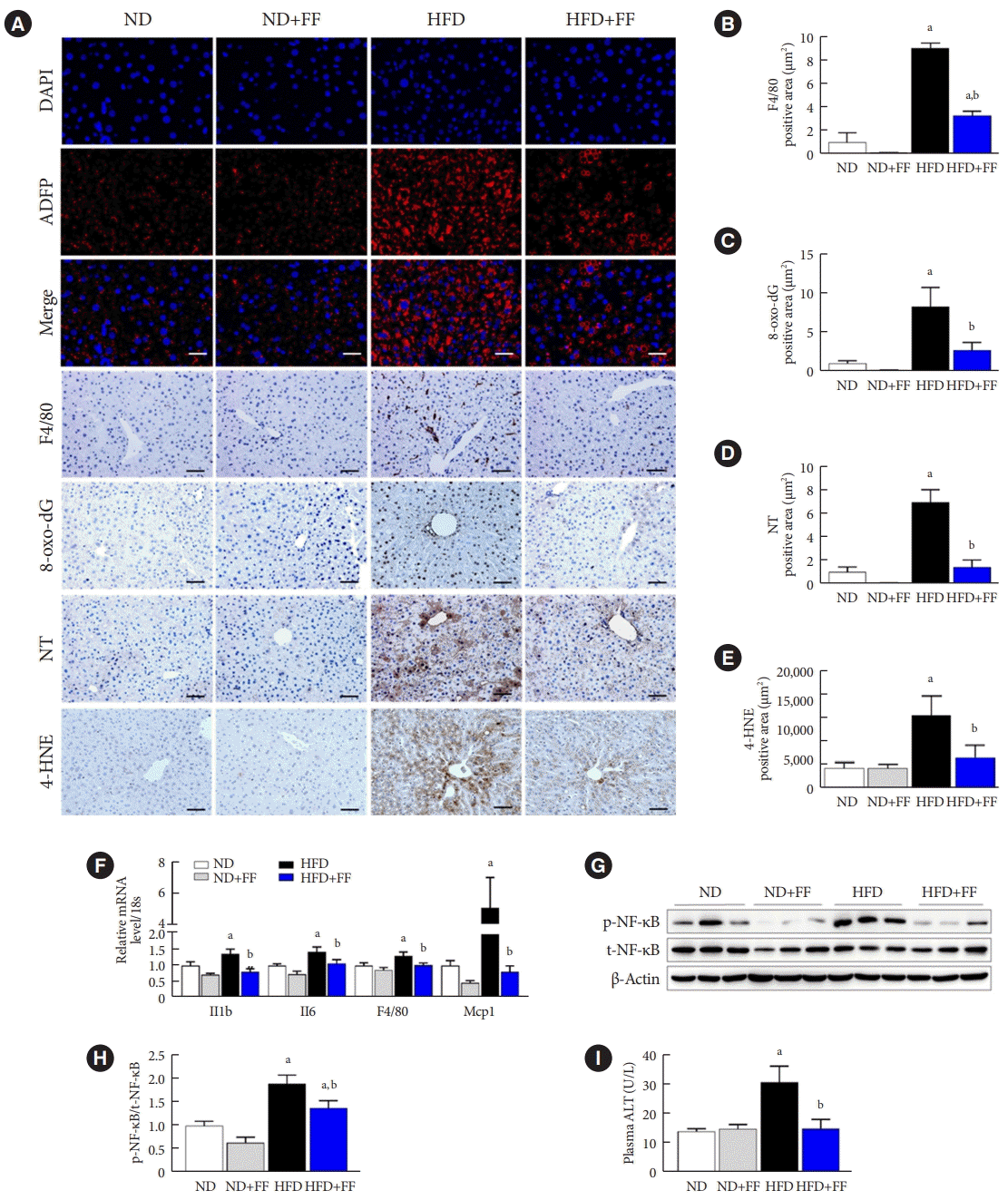
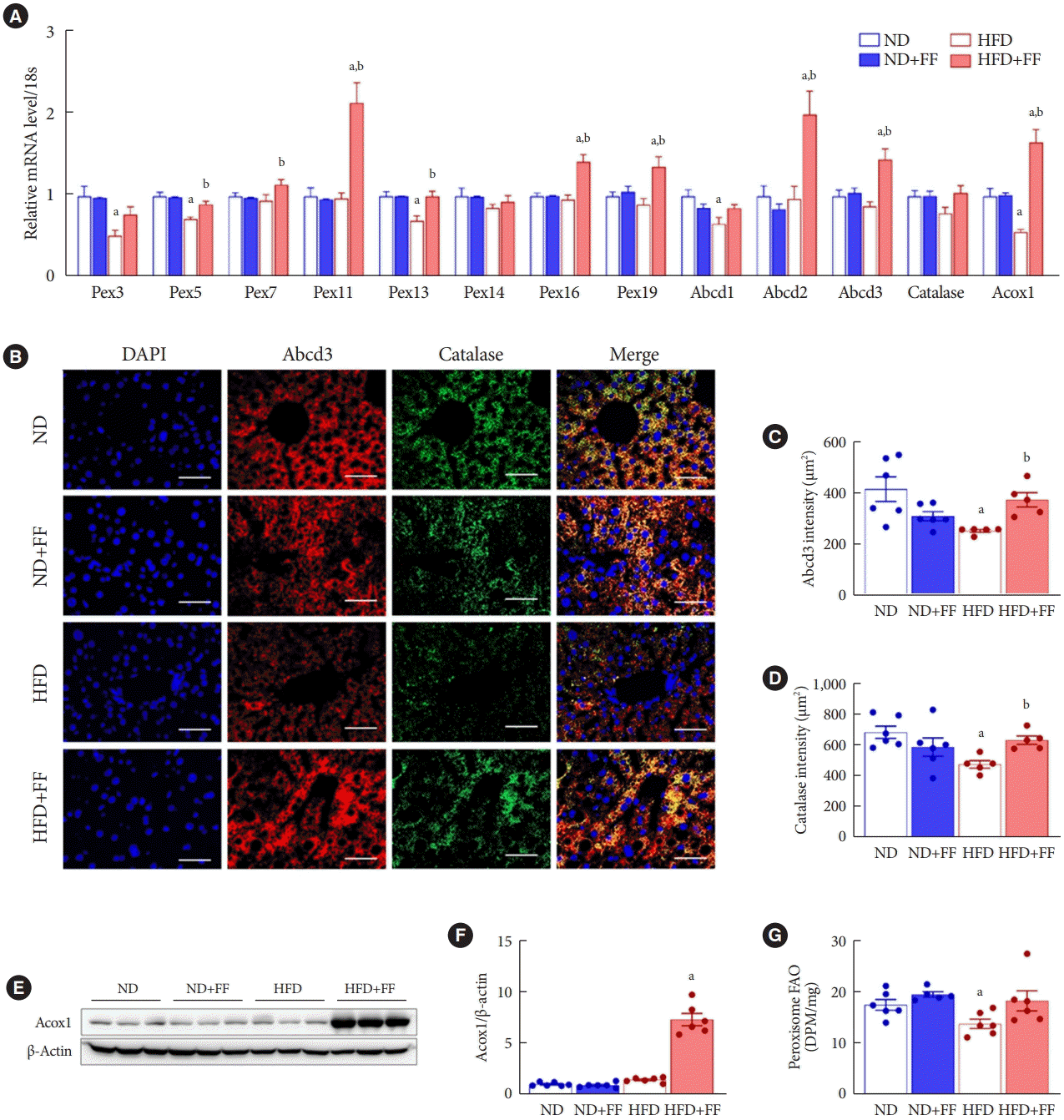
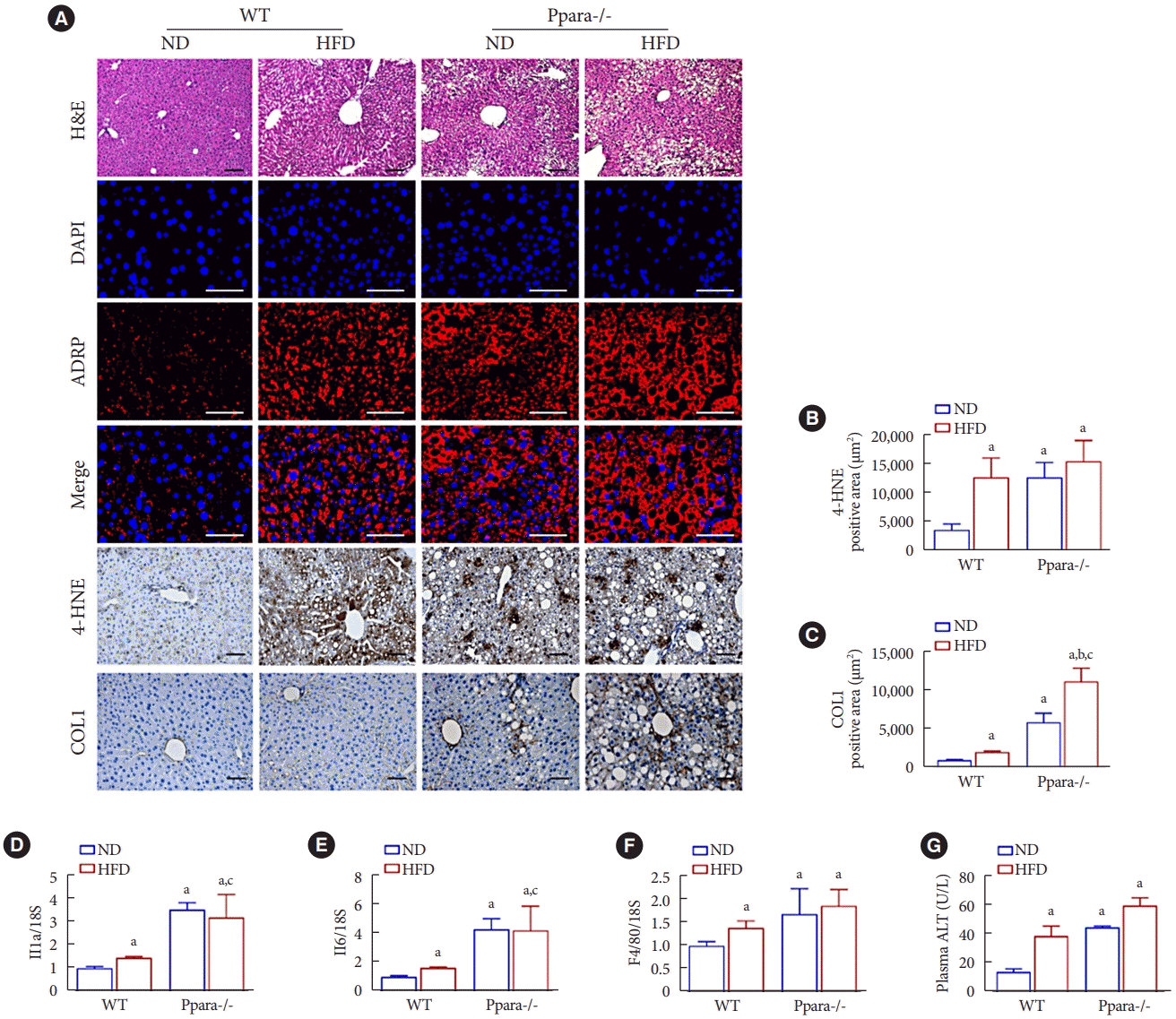
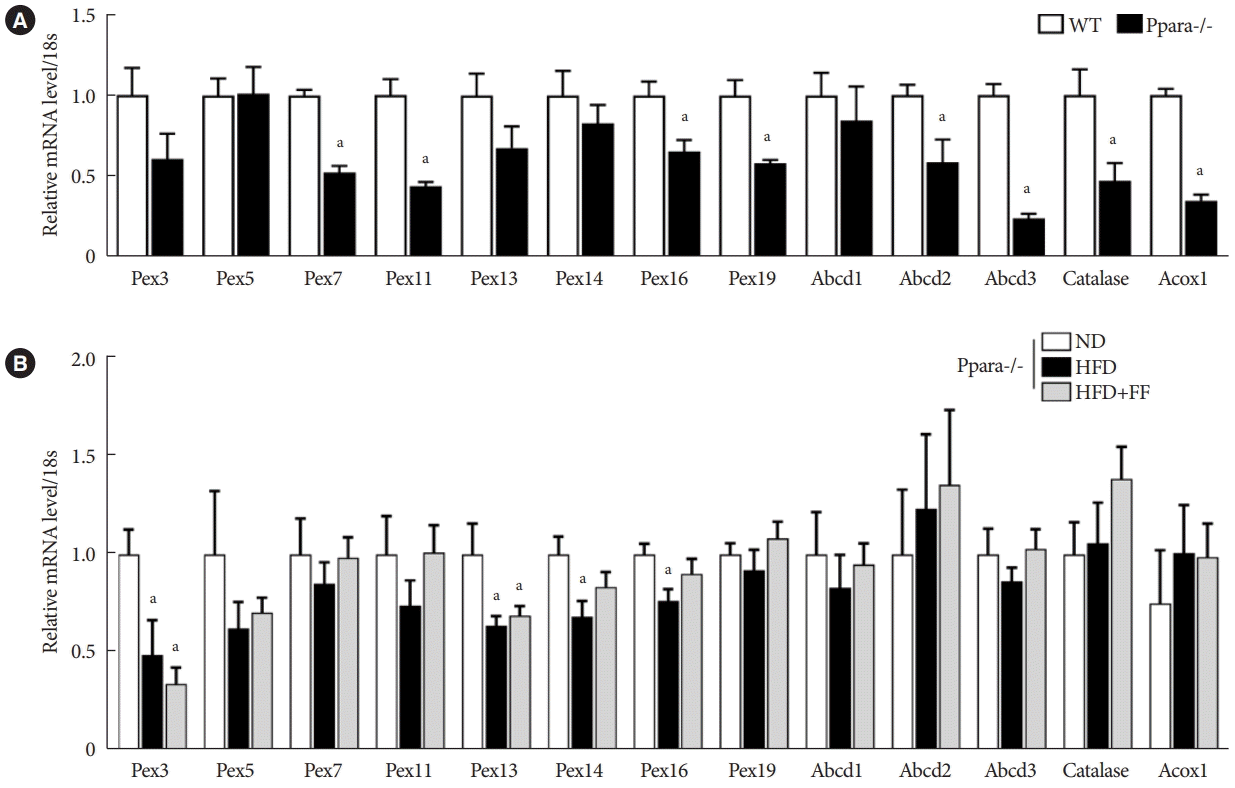




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download