INTRODUCTION
MATERIALS AND METHODS
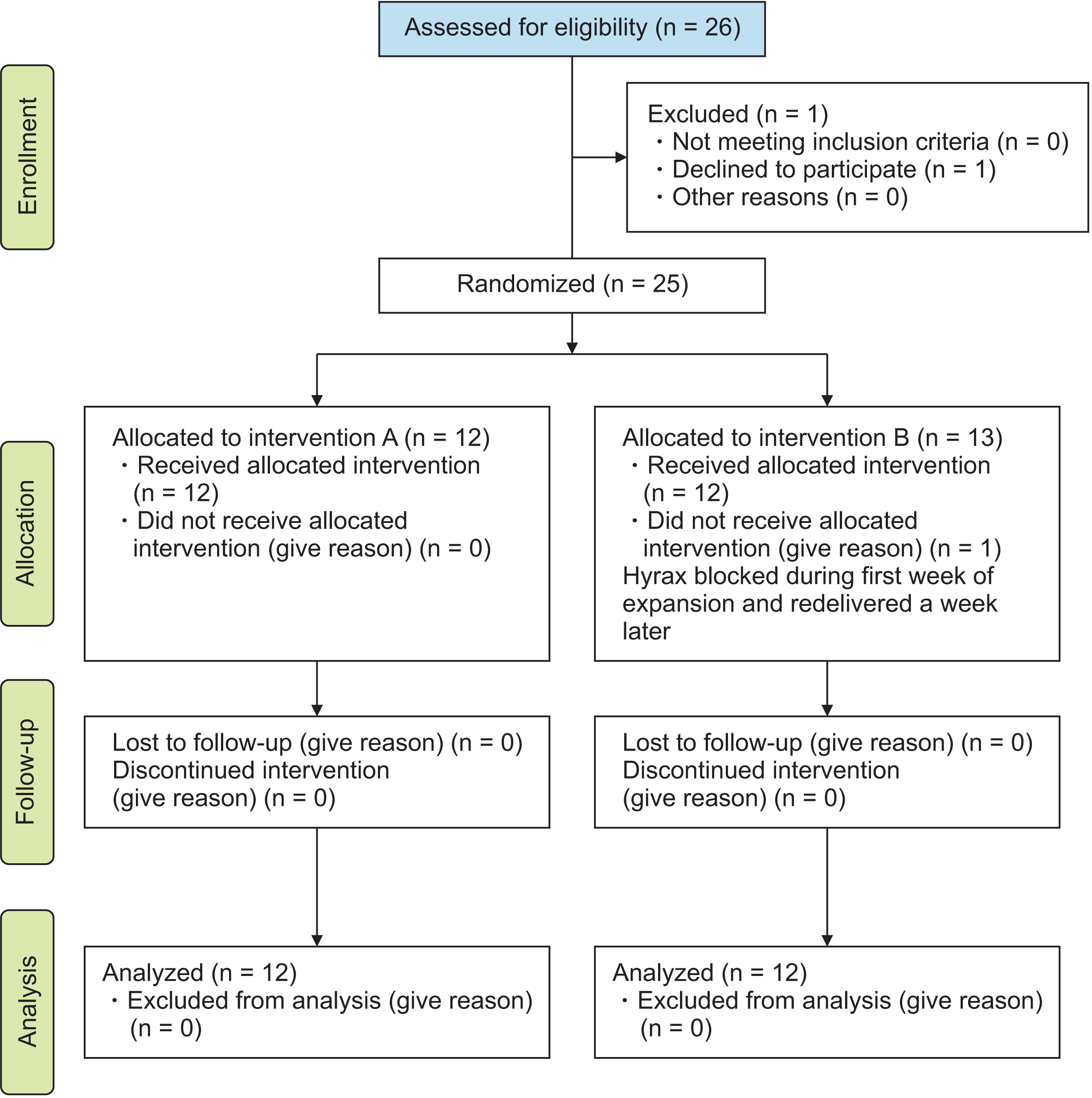
Trial design
Participants, eligibility criteria, and settings
Intervention
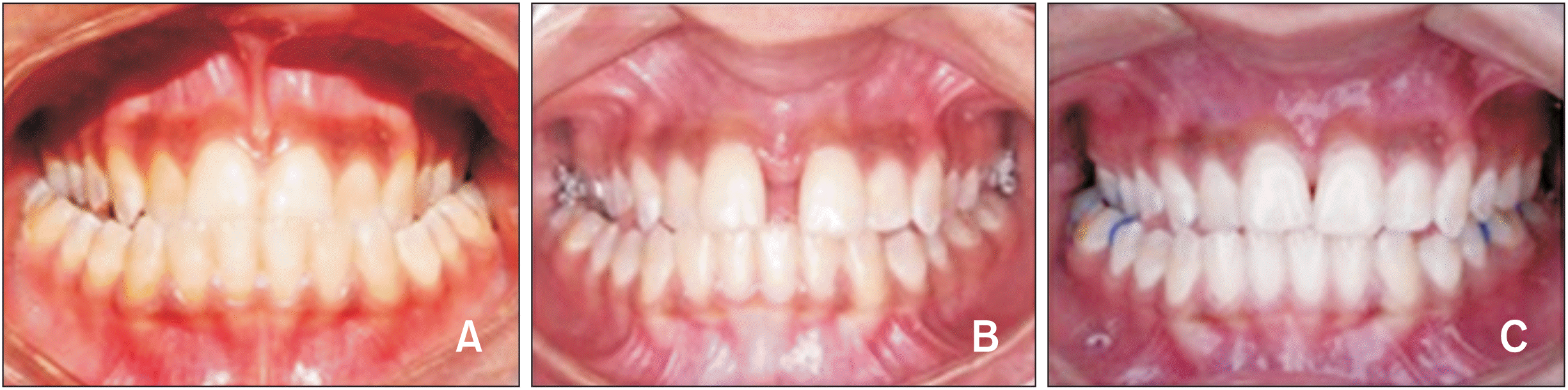
Records
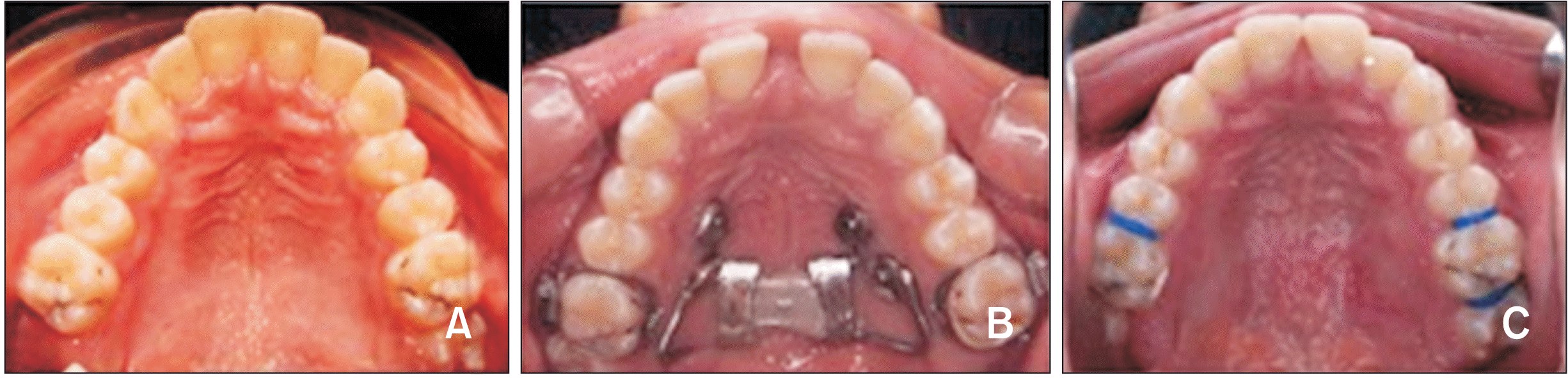
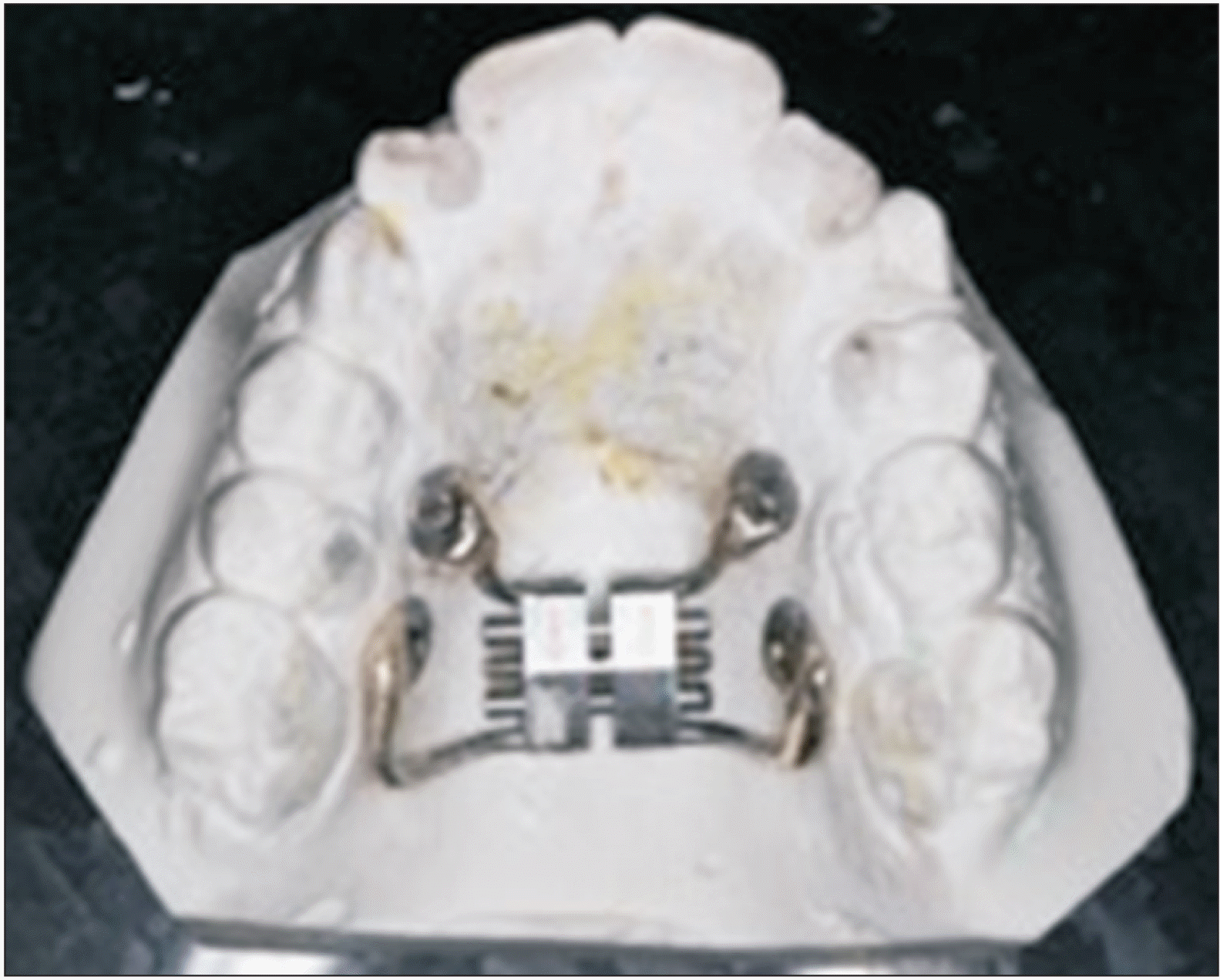
Bone-borne Hyrax expander
LLLT application
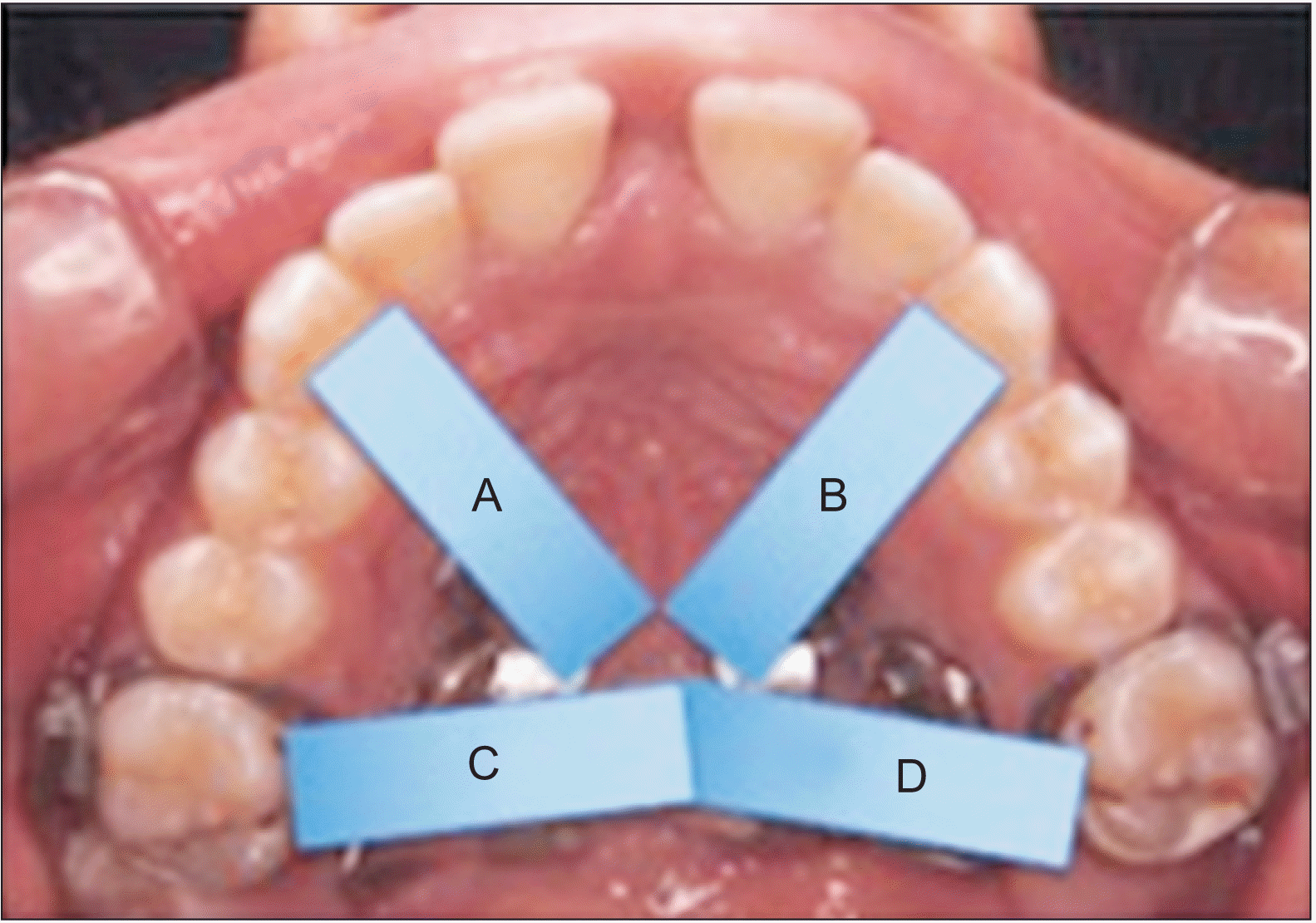
○ Two anterior areas from jackscrew to the canines.
○ Two posterior areas from the jackscrew to the first molars.
○ Day 1 to 5 (first 5 days of expansion).
○ Day 16 to 18 (day 1, 2, and 3 of the retention phase).
○ Day 25 (7 days after the previous dose).
○ Day 32 (7 days after the previous dose).
○ Day 39 (7 days after the previous dose).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download