INTRODUCTION
MATERIALS AND METHODS
Preparation of specimens
Surface treatment
Surface analysis
In vitro test
In vivo test
Statistical analysis
RESULTS
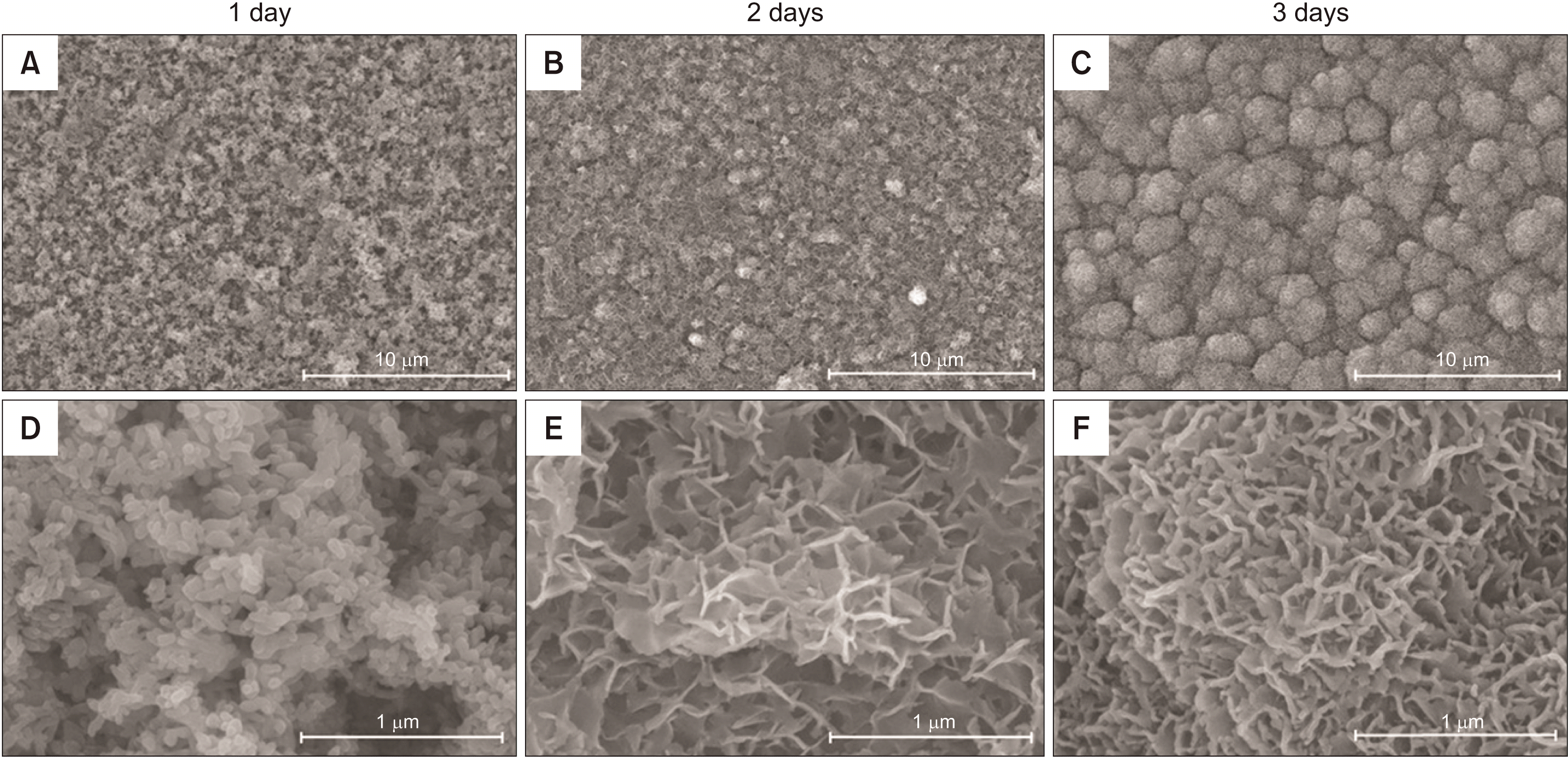
Bioactivity analysis by immersion in SBF (Figure 1, Table 2)
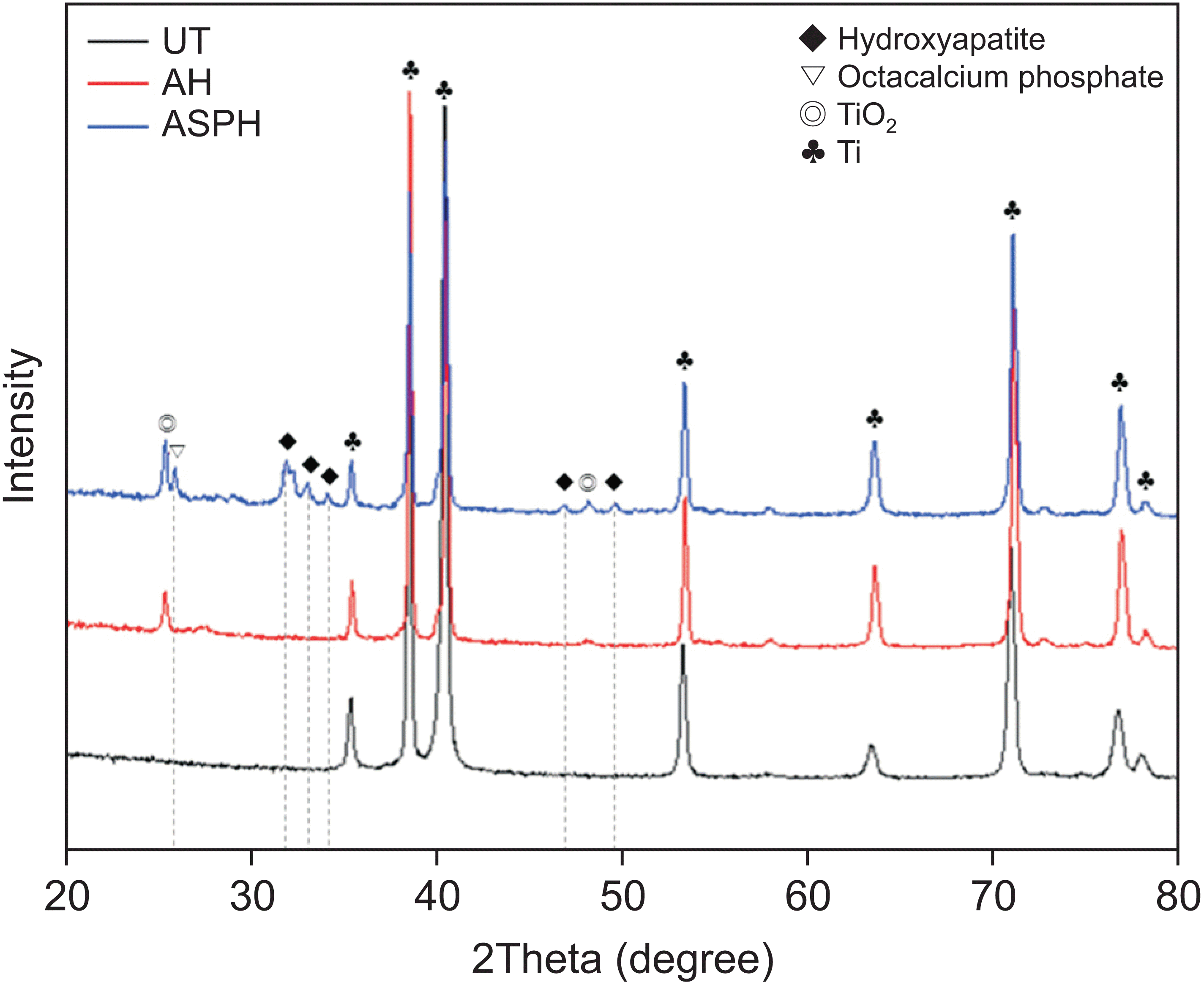
XRD analysis (Figure 2)
Cell cytotoxicity test (Figure 3)
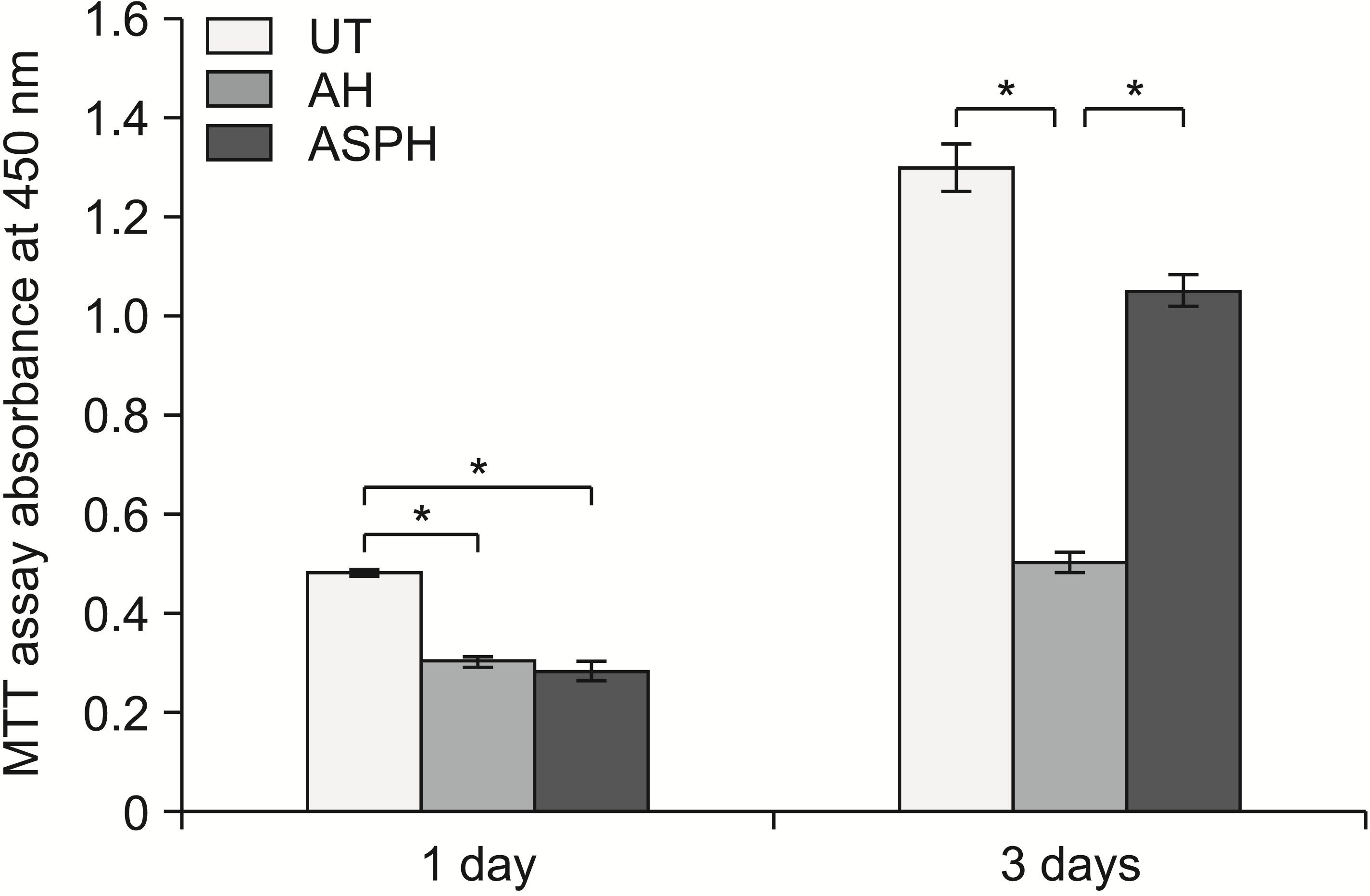 | Figure 3Cytotoxicity test of MC3T3-E1 osteoblast cells in the UT, AH, and ASPH groups for 1 and 3 days (MTT assay). The results are presented as the means ± standard deviation.
UT, untreated group; AH, anodized and heat-treated group; ASPH, anodized treatment followed by cyclic pre-calcification group; MTT, methylthiazolyldiphenyl-tetrazolium bromide.
*p < 0.05.
|
Measurement of removal torque and surface analysis of removed mini-screws (Figures 4 and 5)
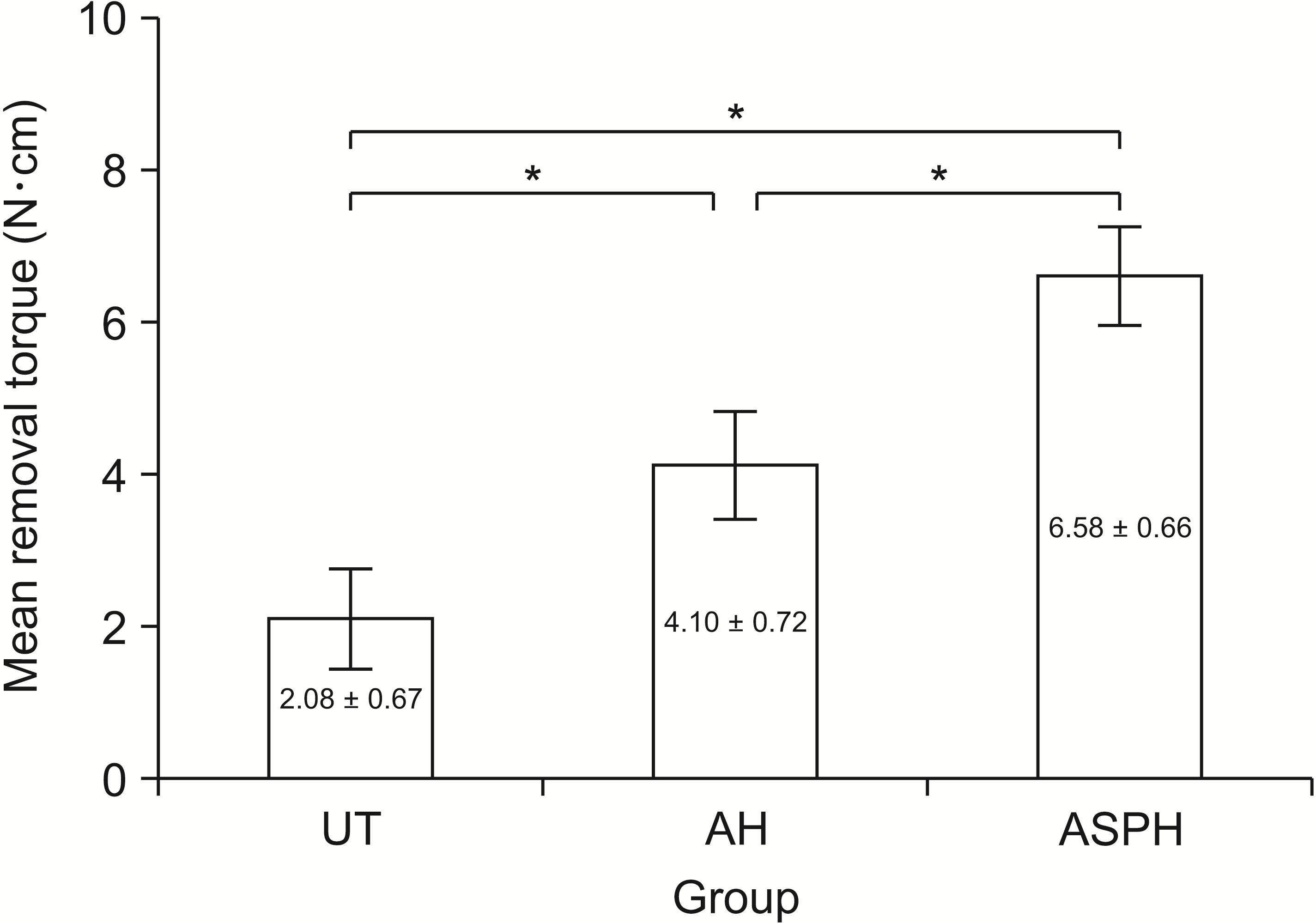 | Figure 4Mini-screws removal torque values of the UT, AH, and ASPH groups at 4 weeks after implantation. The results are presented as the means ± standard deviation.
UT, untreated group; AH, anodized and heat-treated group; ASPH, anodized treatment followed by cyclic pre-calcification group.
*p < 0.05.
|
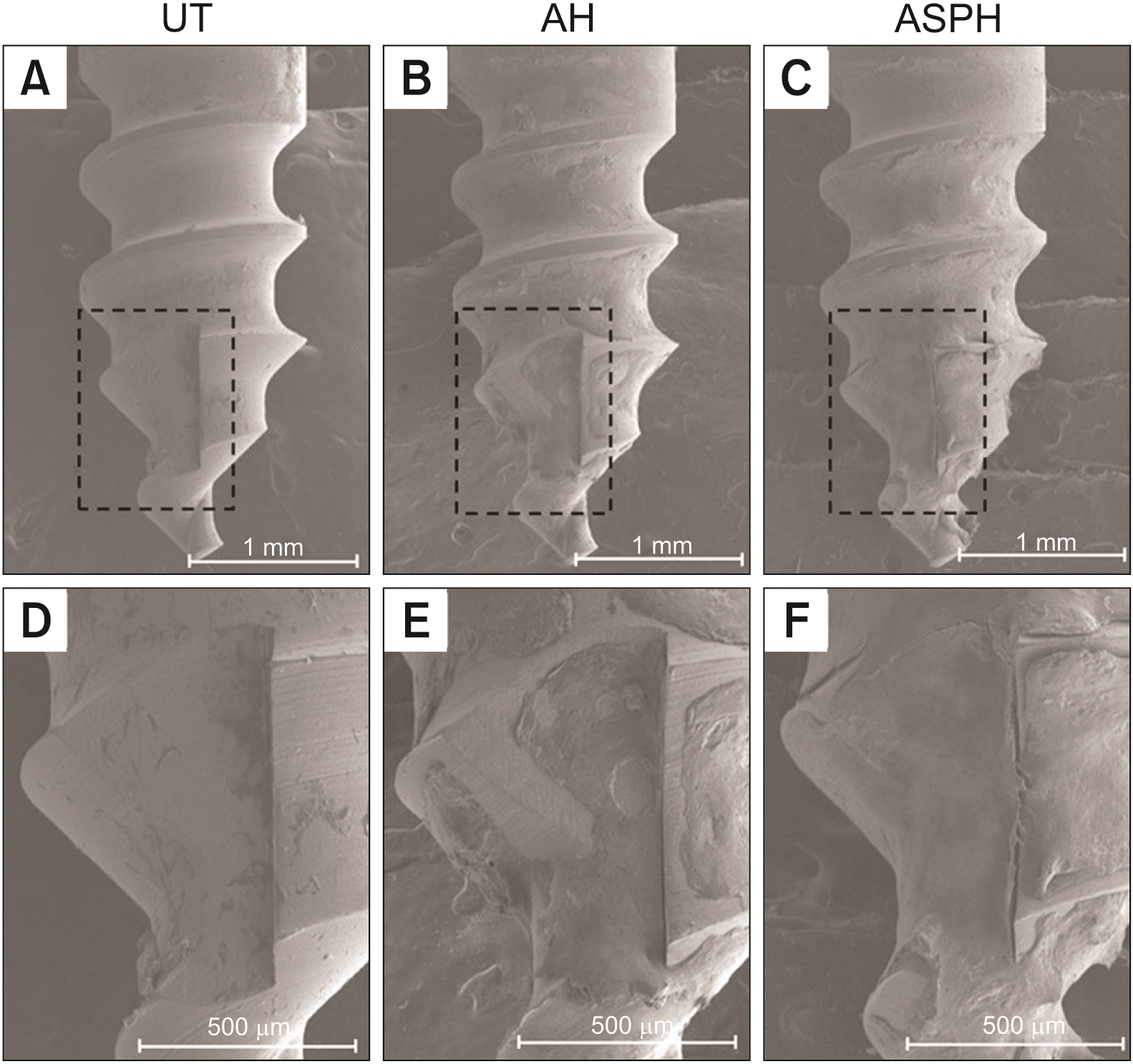 | Figure 5Surface morphology (×40) of mini-screws removed after implantation in rat tibia for 4 weeks. UT (A, D), AH (B, E), and ASPH (C, F) groups. The black squares show high-magnification images (×100).
UT, untreated group; AH, anodized and heat-treated group; ASPH, anodized treatment followed by cyclic pre-calcification group.
|
Histomorphometric analysis (Figure 6)
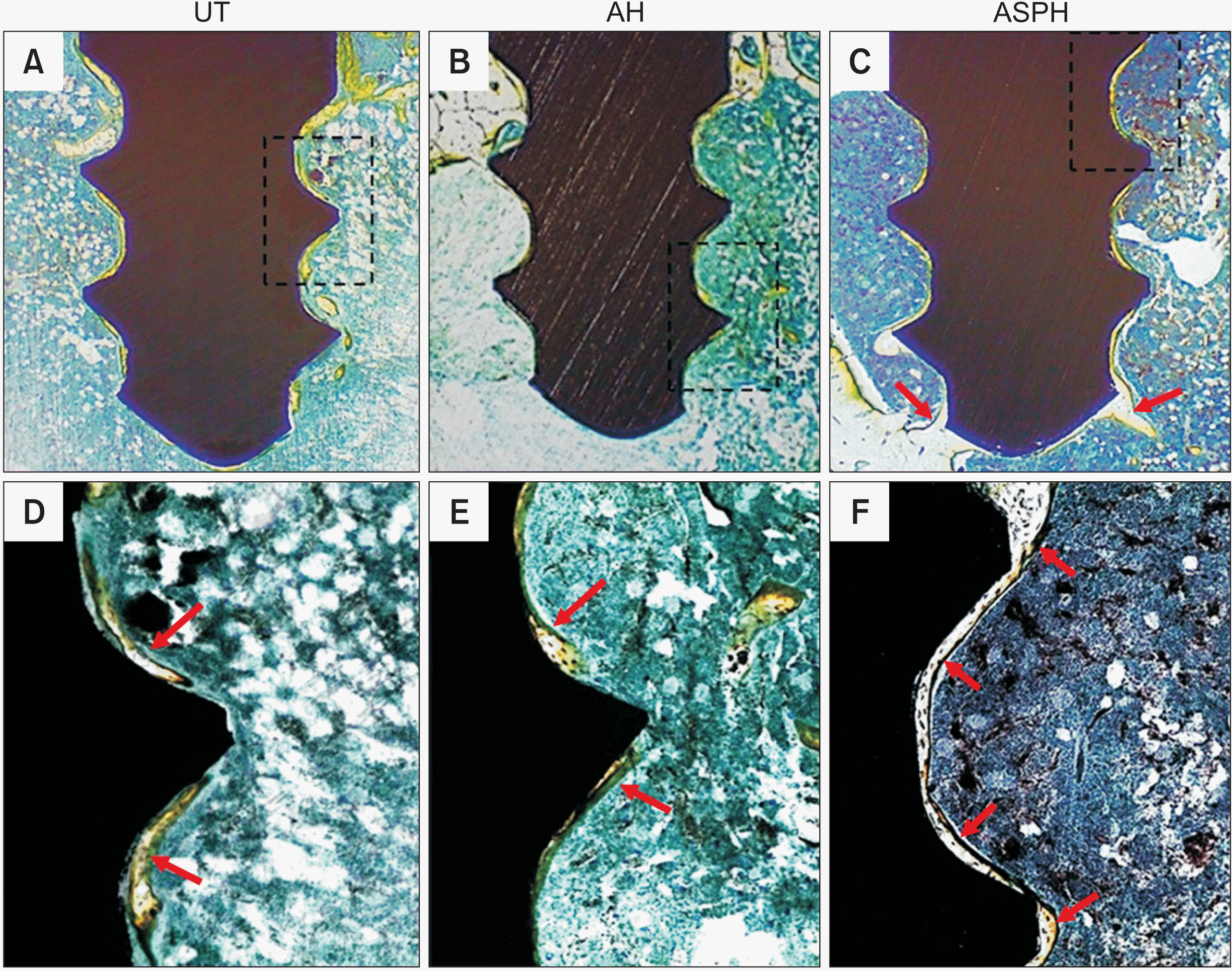 | Figure 6Optical microscope analysis (×30) after 4 weeks of mini-screws implantation. Surface morphology of UT (A, D), AH (B, E), and ASPH (C, F) group mini-screws removed after implantation in rat tibia for 4 weeks. The black squares show high-magnification images (×100). The red arrow indicates osteogenesis.
UT, untreated group; AH, anodized and heat-treated group; ASPH, anodized treatment followed by cyclic pre-calcification group.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download