Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin’s lymphoma. Although progressive lymphadenopathy is a typical feature, extranodal involvement may also occur, including the gastrointestinal tract, skin, bone, thyroid, and testes. Central nervous system invasion is rare, so differentiating it from diseases such as inflammatory demyelinating disorder or infection is essential. DLBCL is therefore a challenge to diagnose, especially when the first findings are neurological symptoms. We report an unusual case of DLBCL that presented as transverse myelitis.
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin’s lymphoma (NHL), and is a disease with rapid progression.1 It can involve one or more organs and occur in lymphoid or extralymphoid sites.2 Reportedly 25-40% of DLBCL cases originate in tissues other than lymph nodes, with the gastrointestinal tract being the most common; involvements of the skin, bone, thyroid, testes and central nervous system (CNS) have also been reported.3 CNS involvement is uncommon and often fatal, mostly due to secondary involvements including CNS relapse or invasion with systemic progression rather than primary or isolated involvements (Table 1).4 Identifying lymphoma as the cause of neurological deficit is a diagnostic challenge when the clinical symptoms are atypical. Here we report a patient who presented transverse myelitis (TM) as the first manifestation of DLBCL without other clinical features.
A 56-year-old male with a past medical history of hypertension presented to the neurology department with acute-onset progressive paresthesia of the right leg, weakness in both lower extremities, and voiding dysfunction for 10 days. He did not complain of systemic symptoms such as weight loss, fever, chill, or night sweats, and there was no evidence of enlarged lymph nodes. The findings of a neurological examination were consistent with weakness in two limbs, with a Medical Research Council grades of 4 and 3 in the proximal and distal aspects of both lower extremities, respectively. Sensation was present at the T12 level, and deep tendon reflexes were absent in the lower extremities and normal in the upper extremities. Hoffman and Babinski signs were absent. Cortical and cranial nerve function tests produced normal results. There were no remarkable findings in a physical examination other than decreased anal tone. Laboratory tests were normal, including full blood count, urine analysis, thyroid function, liver and renal function, lactate dehydrogenase (LDH) (244 U/L; normal range, 0-250 U/L), β2-microglobulin (2.0 mg/L; normal range, 0-2.4 mg/L), and tumor markers. Serological investigations of autoimmune antibodies including paraneoplastic antibodies did not reveal any abnormalities. Human immunodeficiency virus and syphilis antibodies were seronegative. A cerebrospinal fluid examination presented an opening pressure of 125 mmH2O. Both red and white blood cell counts were 0/mm3, and protein concentration was elevated at 75 mg/dL (< 45 mg/dL) without any atypical or malignant cells. No oligoclonal bands were detected. Antiaquaporin 4 and antimyelin oligodendrocyte glycoprotein antibodies were absent in the serum. Magnetic resonance imaging (MRI) of the whole spine indicated mild cord swelling with diffuse T2-weighted hyperintensity without abnormal enhancement at the T11 and T12 levels, including at the conus medullaris (Fig. 1A, B). Brain MRI produced normal findings.
The patient immediately started receiving 1 g of methylprednisolone intravenously for the first 5 days, then intravenous immunoglobulin (IVIG) at 2 g/kg over 5 consecutive days. However, his condition gradually deteriorated, sensory symptoms extended to the left side, bowel incontinence developed, and he became unable to walk independently. Repeated spine MRI revealed a longitudinal extensive lesion between T7 and T12 with diffuse T2-weighted hyperintensity (Fig. 1C, D). A palpable painless mass presented in the right testis 5 days later. Computed tomography (CT) scans of the abdomen and pelvis demonstrated right testicular enlargement with heterogeneous enhancement, which suggested lymphoma (Fig. 2A). Right orchiectomy was therefore performed. Histological sections presented a tumor that had replaced the testis parenchyma (Fig. 2D). On immunohistochemical staining, cells presented strong and diffuse positivity for CD20 (Fig. 2E). Fluorodeoxyglucose (FDG) positron-emission tomography/CT revealed an intensely FDG-avid lymph node in the aortocaval area (Fig. 2B, C). The final diagnosis was DLBCL, and chemotherapy was started. The patient’s initial neurological deficits only partially improved, and he is currently undergoing a combination of rehabilitation and chemotherapy.
DLBCL is a rapidly growing type of NHL that does not always include typical lymphadenopathy and can occur in almost any tissue.2 Although DLBCL is an aggressive lymphoma, it is considered to be treatable.2 However, it has been reported that treatment is difficult and the prognosis is poor in cases of CNS invasion, which is rare among extranodal involvements.1 TM in lymphoma can be caused by the direct involvement mechanism of the spinal cord or immunogenic paraneoplastic syndrome.5 There are two mechanisms for the direct infiltration of tumor cells into the spinal cord.5 The first is invasion into the spinal cord itself, which is possible through epidural, intradural-extraspinal, and intraspinal lymphoma metastases.5 The second is infiltration through the presence of malignant lymphocytes in the lumen of the contributory small and medium-size vessels.5 This results in vascular occlusion, which in turn leads to ischemic, hemorrhagic, or necrotic lesions.6 This mechanism, called intravascular lymphoma, is known to mostly occur in the skin and CNS.7 Paraneoplastic syndrome can present as TM through various underlying pathological mechanisms including inflammation, demyelination, and necrosis, which are associated with immunological response disturbance via cytotoxic T-cell reactions and the overproduction of cytokines and some hormones.5
DLBCL is characterized by systemic symptoms and the painless enlargement of lymph nodes. Blood tests presented blood count abnormalities and elevated serum LDH. To confirm DLBCL diagnosis, the pathological findings must be identified.1 TM can arise from various causes, but often occurs as an autoimmune reaction following an infection or vaccination, or as a result of direct infection, an underlying systemic autoimmune disease, or demyelinating diseases.8 When TM is the first clinical finding of lymphoma without other evidence, possible causative diseases should be excluded and histological findings are essential factors for confirming the diagnosis, but performing a CNS biopsy is not easy. It can therefore be very difficult to diagnose DLBCL in the absence of other systemic involvements. However, if TM is identified with unclear and atypical etiology, lymphoma (particularly intravascular lymphoma) should be considered, and (especially in the elderly) obtaining clinical evidence through random skin biopsy or bone marrow study may play an important role in the diagnosis; CSF examination by flow cytometry may also be helpful.9,10 Early diagnosis allows prompt access to appropriate chemotherapy options, and in turn leads to a more favorable outcome.6
Our patient presented myelopathy as the first finding of DLBCL, and was finally diagnosed 3 weeks later. His initial full blood count and serum LDH level were normal, CSF cytology was negative, and other typical clinical symptoms did not appear until a testicular mass was found. Since we did not identify the pathology of the spinal cord lesion and could not measure paraneoplastic syndrome antibodies, we also could not confirm the exact mechanism. However, the lack of evidence for the other more common causes of myelopathy, ineffectiveness of steroids and IVIG, and rapid progression of clinical deficits suggested that TM development is an extension of the pathophysiological potential of DLBCL. This case suggests that TM can occur as the first manifestation of DLBCL. It highlights that lymphoma involving the spinal cord can be misdiagnosed as a demyelinating disease. It therefore is necessary to consider lymphoma as a cause in the differential diagnosis of TM if other diseases are excluded.
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (No. 2022R1A2B5B01001933) and by the Biomedical Research Institute Fund, Jeonbuk National University Hospital.
REFERENCES
1. Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021; 384:842–858.
2. Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018; 50:74–87.
3. Shi Y, Han Y, Yang J, Liu P, He X, Zhang C, et al. Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: analysis of 1,085 WHO classified cases in a single institution in China. Chin J Cancer Res. 2019; 31:152–161.
4. Chen Y, Lin C, Zhang B. Non-Hodgkin lymphoma with longitudinally extensive transverse myelopathy as the initial symptom: a case report. Front Oncol. 2019; 9:266.
5. Kim HS, Kim KK, Kim OJ. A case of diffuse large B-cell lymphoma presenting as transverse myelopathy. J Neurocrit Care. 2008; 1:177–180.
6. Kumar N, Keegan BM, Rodriguez FJ, Hammack JE, Kantarci OH. Intravascular lymphoma presenting as a longitudinally-extensive myelitis: diagnostic challenges and etiologic clues. J Neurol Sci. 2011; 303:146–149.
7. Ferreri AJ, Campo E, Seymour JF, Willemze R, Ilariucci F, Ambrosetti A, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004; 127:173–183.
8. Frohman EM, Wingerchuk DM. Clinical practice. Transverse myelitis. N Engl J Med. 2010; 363:564–572.
9. Tolvaj B, Hahn K, Nagy Z, Vadvári Á, Csomor J, Gelpi E, et al. Life threatening rare lymphomas presenting as longitudinally extensive transverse myelitis: a diagnostic challenge. Ideggyogy Sz. 2020; 73:275–285.
10. Yunoki M, Suzuki K, Uneda A, Yoshino K. A case of intravascular lymphoma presenting as myelopathy diagnosed with a skin biopsy. Surg Neurol Int. 2015; 6(Suppl 13):S367–S370.
11. Merino A. Diffuse large B cell lymphoma presenting as transverse myelitis. Minn Med. 2015; 98:46.
12. Shea L, Zhao Y, Reddy V, Yacoubian T, Mehta A. Primary bone marrow diffuse large B-cell lymphoma presenting as transverse myelitis. Am J Med Sci. 2018; 356:561–566.
13. Kim H, Nam TS, Levy M, Lee KH, Kim J, Lee SJ. Primary central nervous system lymphoma with intramedullary spinal cord involvement mimicking inflammatory demyelinating disease. J Neurocrit Care. 2019; 12:55–63.
Fig. 1.
Magnetic resonance imaging of the spine was performed initially and 15 days after symptom onset. Initial T2-weighted (A) and postcontrast T1-weighted (B) images present mild cord swelling with T2-weighted hyperintensity without enhancement at the T11 and T12 levels (white arrow). Follow-up T2-weighted image (C) and gadolinium-enhanced T1-weighted image (D) demonstrate longitudinally extensive lesions over six vertebral segments (T7 to T12), with accompanying diffuse enhancement (white arrow).
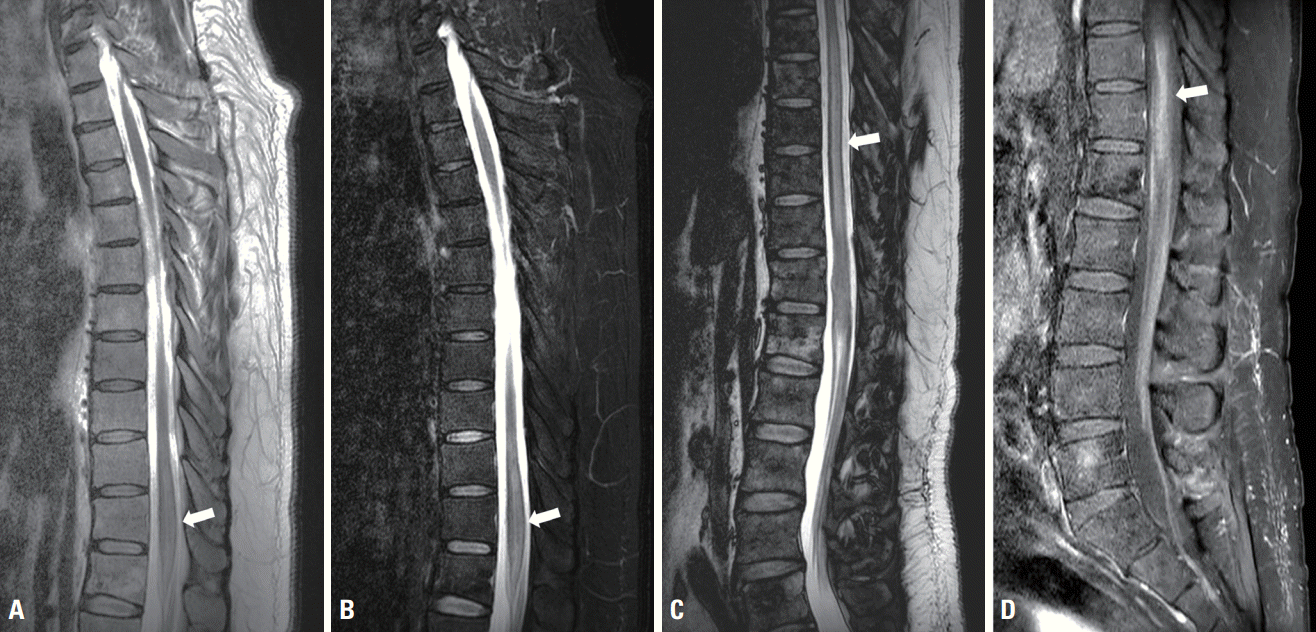
Fig. 2.
Computed tomography (CT) of the abdomen and pelvic cavity revealed swelling of the right testis (arrow) with heterogeneous enhancement (A). Fluorodeoxyglucose (FDG) positron-emission tomography/CT performed after right orchiectomy demonstrating mild diffuse hypermetabolism (arrow) thought to be due to a postoperative change in the right inguinal area (B) and an intense FDG-avid small lymph node (arrow) in the aortocaval area (C). The pathological findings demonstrate large atypical lymphocytes replacing the testis (D; Hematoxylin and Eosin staining, ×200) and tumor cells expressing CD20 (a B-cell marker), with the Ki-67 labeling index approaching 90% (E; Immunohistochemical stain, ×400).
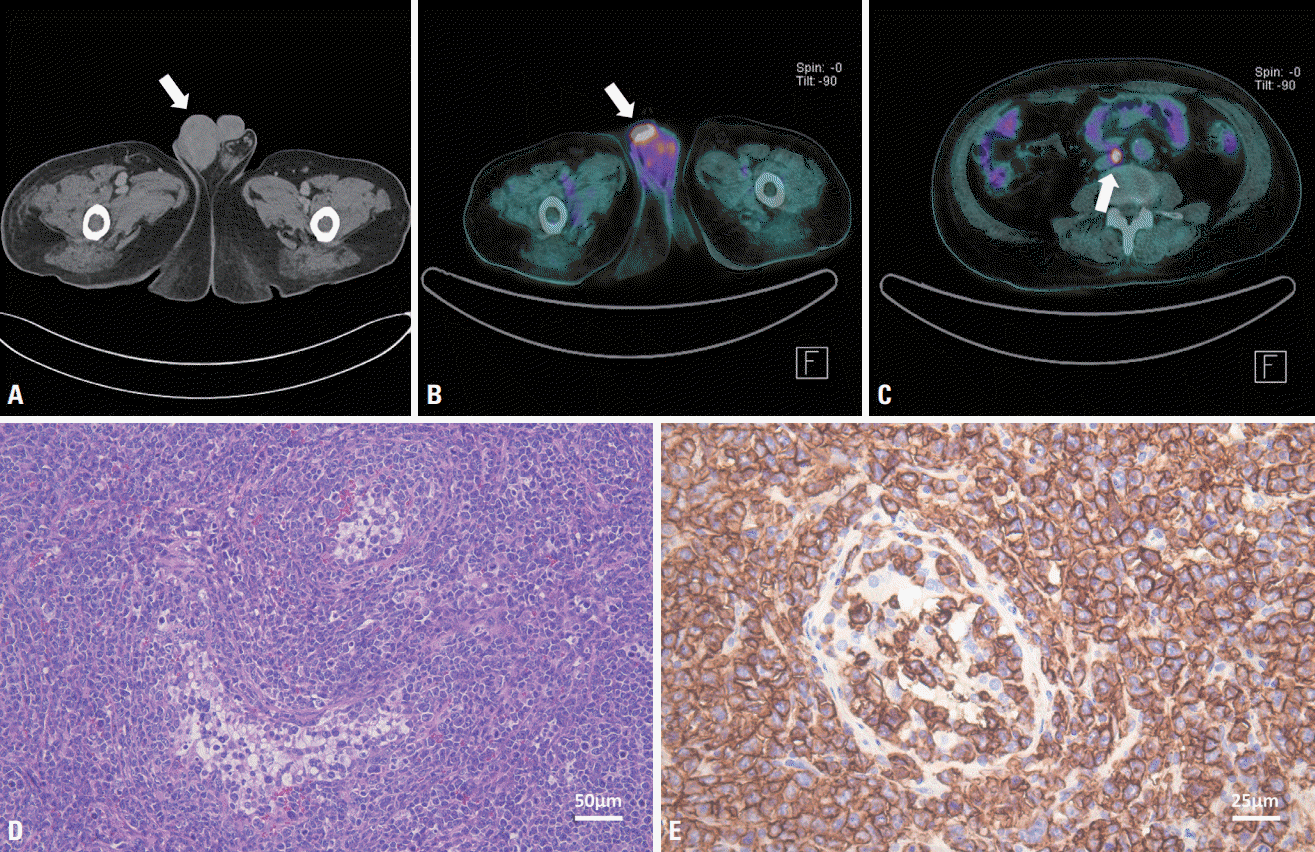
Table 1.
Reported cases of diffuse large B cell lymphoma presented with transverse myelitis as a first manifestation
| Study | Age (years) | Sex | Neurologic symptoms | MRI findings | Final diagnosis | The period from TM to DLBCL diagnosis | Treatment |
|---|---|---|---|---|---|---|---|
| Kim et al. 5 (2008) | 78 | F | Ascending sensory loss, progressive weakness of both legs and dysuria | Poorly delineated increased signal intensity in spinal cord of T2-T4 level on T2 weighted image | Diffuse large B cell lymphoma (lymph node biopsy) | 2 months | The patient did not consent to chemother- apy and died 4 months after the symptom onset |
| Merino 11 (2015) | 64 | M | Leg weakness, urinating difficulty, and sensory deficits bilaterally | Spinal edema at T10 to T12 | Diffuse large B cell lymphoma (liver biopsy) | 4 months | High dose steroid, IVIG, plasmapheresis, and R-CHOP |
| Shea et al. 12 (2018) | 57 | M | Progressive lower extremity weak- ness and bowel and bladder incontinence | Diffusely T2 hyper- intense cord signal involving the terminal cord and conus | Diffuse large B cell lymphoma (bone marrow biopsy) | Several weeks | R-CHOP |
| Chen et al. 4 (2019) | 67 | M | Back pain, progres- sive numbness and weakness of the limbs, and severe quadriparesis | Longitudinally exten- sive abnormal signal extending from the cervical to the thoracic cord | Diffuse large B cell lymphoma (brain, bone marrow biopsy) | 6 months | Systemic chemotherapy (methotrexate, rituximab, Ara-C) combined with involved field radiotherapy |
| Kim et al. 13 (2019) | 46 | M | Weakness in both hands | T2-hyperintensity in the medulla oblongata and cervical spinal cord with leptomen- ingeal enhancement and an intraparenchy- mal tadpole-like signal change | Diffuse large B cell lymphoma (brain biopsy) | 5 months | High dose steroid, high-dose methotrexate |
| This case (2022) | 56 | M | Paresthesia of right leg, weakness of both lower extremities and voiding difficulty | Mild cord swelling with diffuse T2 hyperinten- sity and enhancement from T7 to conus medullaris | Diffuse large B cell lymphoma (intratesticular mass biopsy) | 3 weeks | High dose steroid, IVIG, and R-CHOP |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download