This article has been
cited by other articles in ScienceCentral.
Abstract
Primary central nervous system lymphoma (PCNSL) is a type of non-Hodgkin lymphoma confined to the central nervous system. Its diagnosis requires a stereotactic biopsy, which is an invasive procedure. Cerebrospinal fluid (CSF) analysis is less invasive and easier to perform than a stereotactic biopsy. We hereby report a PCNSL case diagnosed using CSF analysis and treated with systemic chemotherapy.
Go to :

Keywords: Lymphoma, Central nervous system, Immunoglobulin gene
Primary central nervous system lymphoma (PCNSL) is a type of non-Hodgkin lymphoma confined to the central nervous system that often occurs in immunosuppressed patients.
1,
2 We report a PCNSL case diagnosed using cerebrospinal fluid (CSF) analysis.
CASE
An 85-year-old female was referred to a neurology department due to altered consciousness. She was initially hospitalized after complaints of hematochezia, and immunohistochemistry was used to diagnose rectal ulcer and cytomegalovirus (CMV). She was treated using intravenous ganciclovir for 7 days. She demonstrated drowsiness soon after hospitalization, which progressively deteriorated.
The past medical history of the patient indicated that she received oral prednisolone for rheumatic arthritis, and thyroid hormones. She had undergone gastrectomy for gastric cancer during the previous year, and had received the Pfizer vaccine for COVID-19 4 months previously.
Her body temperature was 37.1°C, pulse rate was 110 beats/min, and blood pressure was 140/80 mmHg. She had a stupor mental state with no lateralizing signs. Laboratory tests indicated mild anemia (8.3 g/dL), elevated thyroid-stimulating hormone (6.33 mIU/L), and decreased free thyroxine (0.82 ng/dL). Gadolinium-enhanced T1-weighted and fluid-attenuated inversion recovery imaging indicated diffuse leptomeningeal enhancement in the brainstem and nodular contrast enhancement in the ependymal cells of the fourth and lateral ventricles (
Fig. 1).
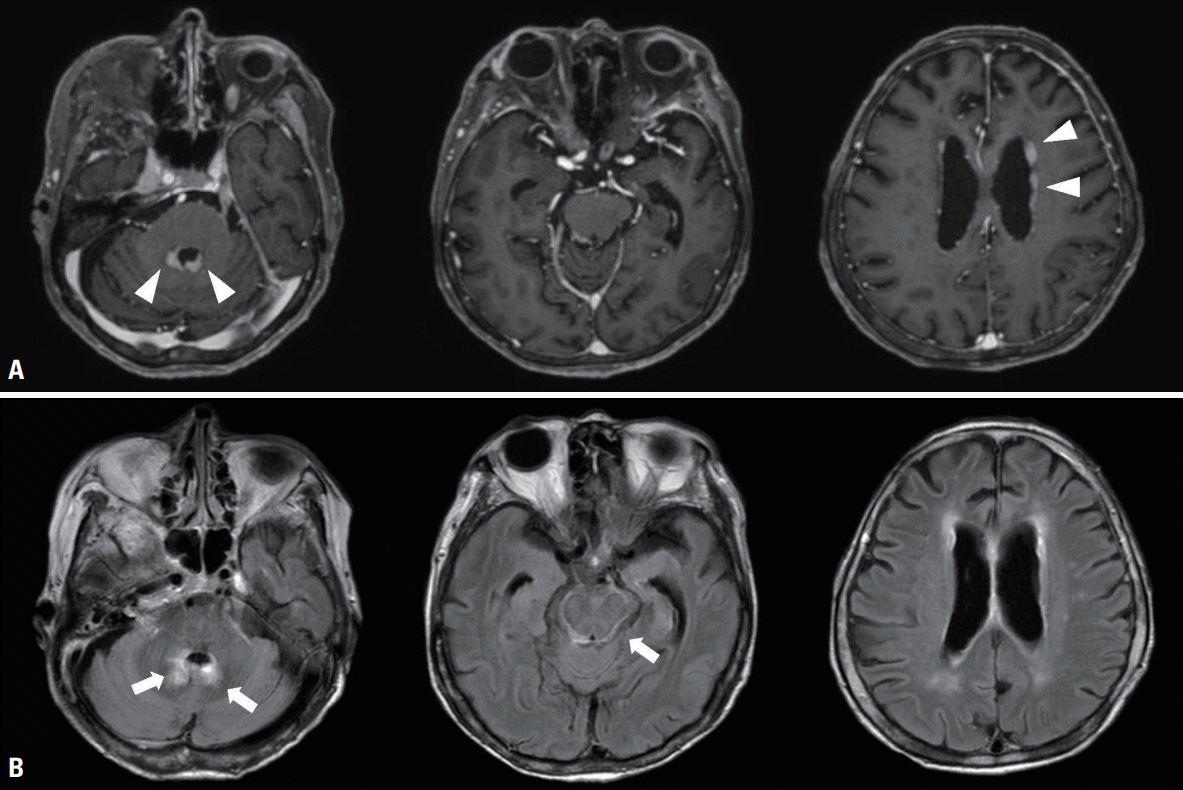 | Fig. 1.(A) Gadolinium-enhanced T1-weighted images showing nodular contrast enhancement in the ependymal cells of the fourth and lateral ventricles (white arrowheads). (B) Gadolinium-enhanced fluid-attenuated inversion recovery images revealing diffuse leptomeningeal enhancement along with brainstem and nodular contrast enhancement in the ependymal cells of the fourth ventricle (white arrows). 
|
The CSF analysis showed lymphocyte-predominant pleocytosis (60/mm3, 95% lymphocytes), elevated protein (131.2 mg/dL) and adenosine deaminase (40 IU/L), and a decreased CSF/serum glucose ratio (41/192 mg/dL). The following laboratory tests produced negative findings: bacteria and fungi culture/staining, tuberculosis and polymerase chain reactions (PCRs) for herpes simplex, enterovirus, CMV, human T-cell leukemia virus, tuberculosis, and Ebstein-Barr virus; and autoantibodies in the serum and CSF including NMDAR, AMPA, DPPX, LGI1, CASPR2, GABA-B, paraneoplastic antibody, antiganglioside antibody, aquaporin-4 antibody, and myelin oligodendrocyte glycoprotein antibody. However, CSF cytology identified atypical lymphoid cells.
Intravenous ganciclovir was maintained for 3 weeks based on the recommendation of a department of gastroenterology, despite repeatedly negative results on qualitative CMV PCR in the CSF analysis. Immunoglobulin treatment at 2 g/kg for 5 days was initiated for possible autoimmune encephalitis, but there was no improvement. Antituberculosis drugs were then administered for suspected tuberculosis meningitis based on the elevated adenosine deaminase in the CSF, subacute onset, and diffuse basal enhancement.
Since the patient was not responsive to intravenous ganciclovir, immunoglobulin, or 3 weeks of antituberculosis medication, we tried other diagnostic techniques. We considered the presence of PCNSL due to atypical cells found during CSF cytology, serial magnetic resonance imaging indicating diffuse leptomeningeal enhancement in the brainstem, and nodular contrast enhancement in the periventricular areas.
Due to her poor medical condition, we conducted a CSF analysis instead of a brain biopsy. Clonality analysis testing for rearrangement of the genes for immunoglobulin heavy chain and immunoglobulin κ light chain (
IGH and
IGK, respectively) was performed on DNA extracted from CSF using the BIOMED-2 clonality assay (IdentiClone; InVivoScribe Technologies, San Diego, CA, USA), which indicated monoclonality (
Fig. 2). Immunohistochemistry indicated that the atypical cells found during CSF cytology were positive for CD20.
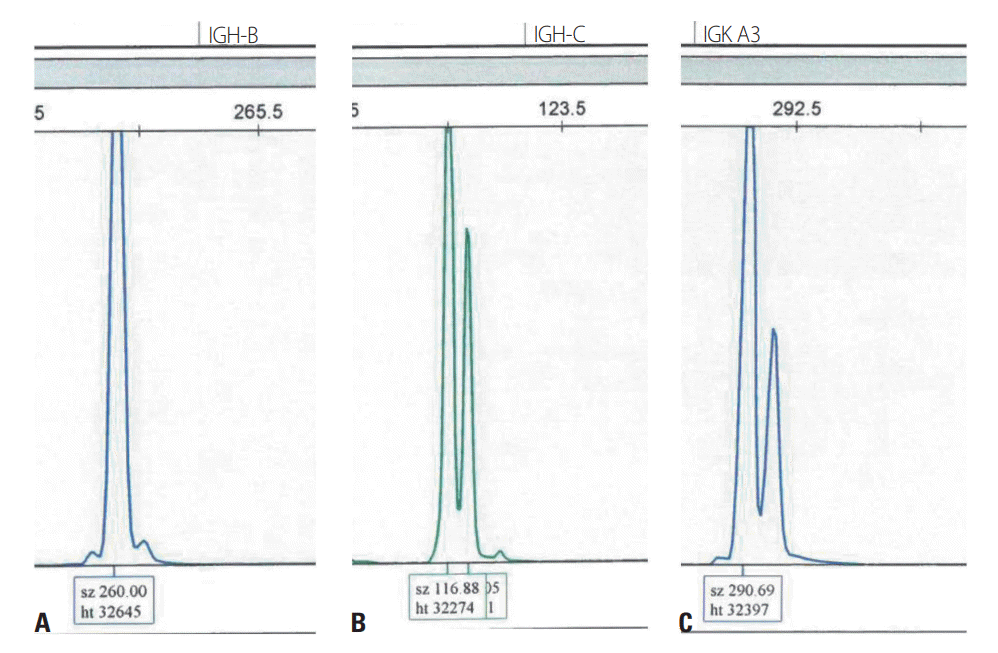 | Fig. 2.Monoclonal rearrangement of immunoglobulin heavy chain (IGH) and immunoglobulin κ light chain (IGK) framework regions in the patient: (A) 260 bp of the IGH-B region, (B) 116 bp of the IGH-C region, and (C) 290 bp of the IGK-A region. 
|
One month after systemic chemotherapy with high-dose methotrexate, her neurologic symptoms and lesions were progressively improved (
Fig. 3).
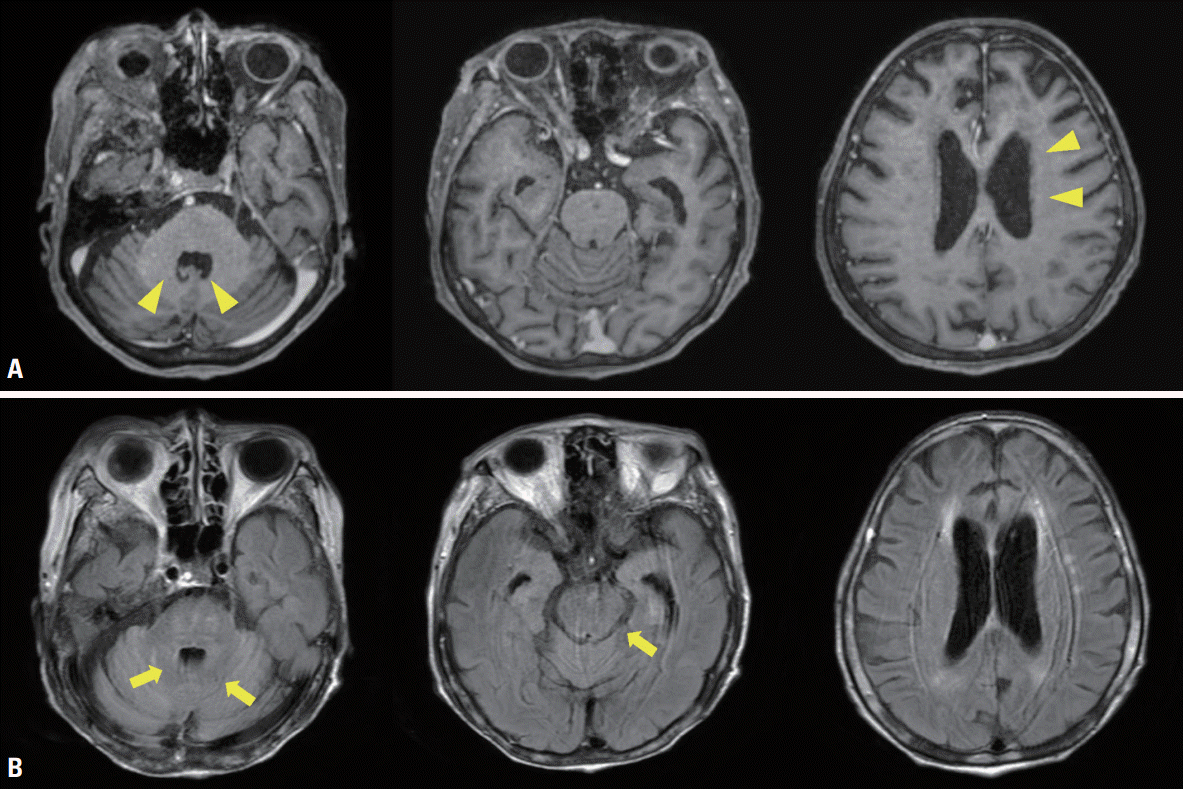 | Fig. 3.(A) Gadolinium-enhanced T1-weighted images indicating improvement of previous nodular enhancement in the periventricular lesion (yellow arrowheads). (B) Gadolinium-enhanced fluid-attenuated inversion recovery images indicating improvement in diffuse leptomeningeal enhancement along with brainstem and nodular contrast enhancement (yellow arrows). 
|
Go to :

DISCUSSION
Diagnosis of PCNSL requires a stereotactic biopsy.
1 However, a brain biopsy is invasive and therefore has several limitations.
3 First, the operation cannot be performed if the patient cannot tolerate anesthesia. Second, other complications such as intracranial hemorrhage and postoperative neurologic deficit can occur. Third, it is difficult to obtain the appropriate specimen if the lesion involves deep brain structures. Fourth, the results of pathologic findings are influenced by previous glucocorticoid therapy.
4 In contrast, CSF analysis is less invasive and easier to perform. For suspected B-cell lymphoproliferative disease, the EuroClonality/BIOMED-2 guidelines recommend testing for immunoglobulin gene rearrangement.
5 Previous studies found that
IGH rearrangement analysis is a useful tool in PCNSL diagnosis.
6,
7 Other investigations found that
IGH rearrangement investigations have a sensitivity of 58% and a specificity of 85% in detecting lymphoproliferative processes.
8 We did not use high-dose steroid pulse therapy due to the presence of massive hematochezia with gastrointestinal ulcer. However, if the patient received intravenous steroid therapy, the influence of glucocorticoid therapy on
IGH rearrangement analysis should be considered and further investigation would be needed. Also, although CSF analysis with immunoglobulin gene rearrangement can be a useful alternative tool, it is risky to diagnose PCNSL using CSF analysis alone without considering clinical manifestations and also other laboratory and imaging findings. The clinician should consider the possibility of other diseases while performing a precise clinical correlation.
When PCNSL is suspected and the patient will not tolerate a brain biopsy, immunoglobulin gene rearrangement and immunohistochemistry should be considered as alternative diagnostic tools for PCNSL.
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download