Abstract
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a chronic recurrent acquired immune-mediated disease of the peripheral nerves that presents with progressive sensory and motor deficits in all four limbs. Cranial nerve involvement is not as common as in Guillain-Barre syndrome, and central nervous system involvement including optic neuritis has rarely been reported in patients with CIDP. We recently experienced a case with classic CIDP involving bilateral facial and trigeminal nerves, right lower cranial nerves, and the right optic nerve.
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a chronic recurrent acquired immune-mediated disease of the peripheral nerves that presents with progressive sensory and motor deficits in all four limbs.1 Cranial nerve involvement is not as common as in Guillain-Barre syndrome, and central nervous system (CNS) involvement including optic neuritis has rarely been reported in patients with CIDP.2,3 We recently experienced a case with CIDP accompanied by acute-onset optic neuritis and multiple cranial neuropathies.
A 42-year-old male presented with bifacial paresthesia and right visual disturbance. He complained of numbness and tingling sensation on both cheeks that first appeared 1 month previously, and also the sudden onset of visual disturbance in the right eye 3 days previously. He did not have ocular pain. He had also suffered from progressive muscle weakness and sensory disturbance in all four limbs for several years, and was diagnosed with classic CIDP 2 years previously, for which he had received immunotherapy with an oral steroid and azathioprine.
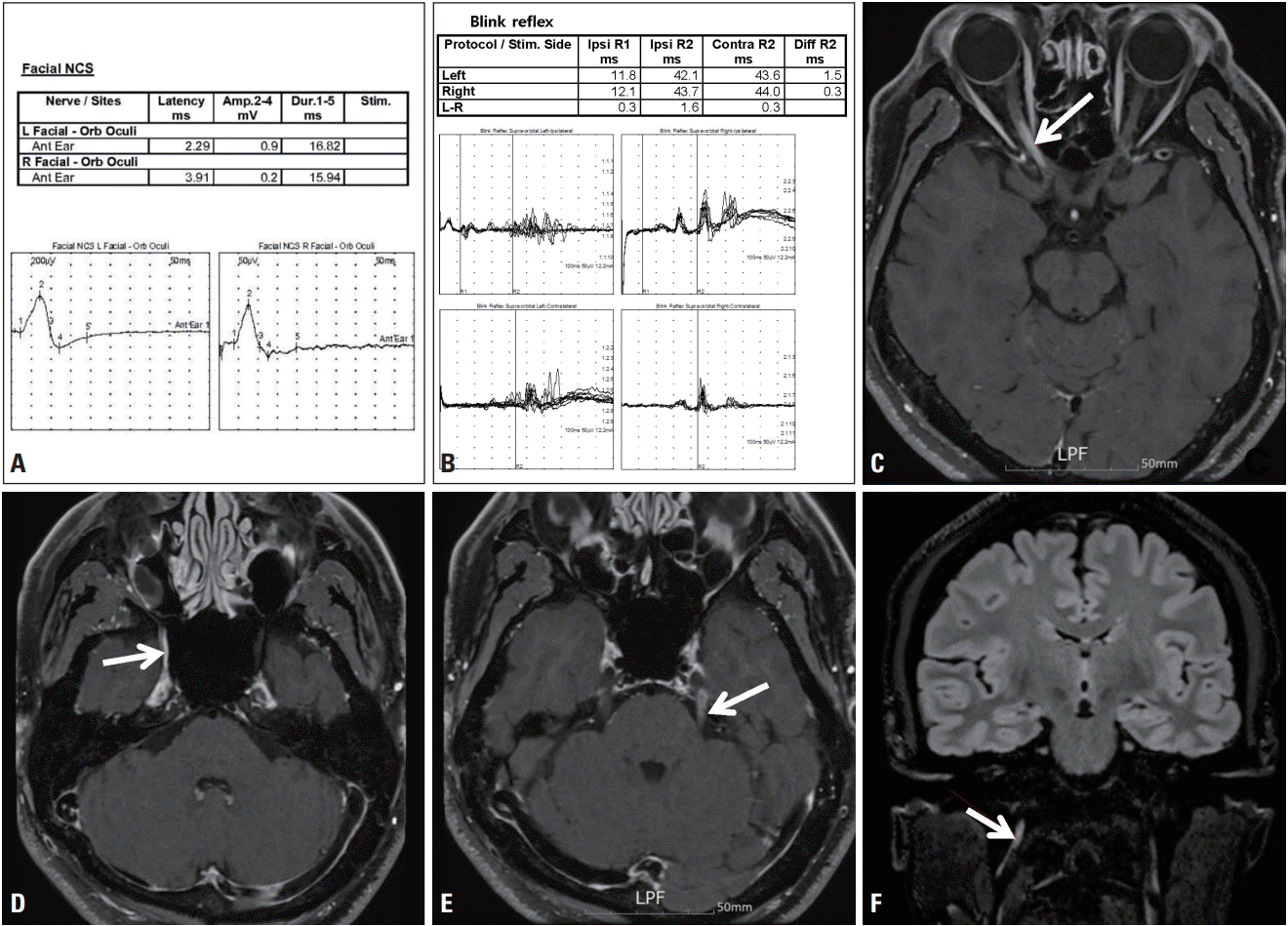
Neurological and ophthalmological examinations revealed hypesthesia, dysesthesia, and hyperalgesia on both cheeks and the chin, and central scotoma and relative afferent pupillary defect in the right eye. Visual acuity was decreased and the color vision test was abnormal on the right side. Funduscopic findings were normal on both sides. Motor nerve conduction studies (NCSs) of both facial nerves revealed delayed terminal latency on the right side and decreased compound muscle action potential (CMAP) amplitudes on both sides (Fig. 1A). The trigeminal somatosensory evoked potential test revealed poor wave formation on both sides. The blink reflex test showed delayed latencies of ipsilateral R1 and R2 waves and contralateral R2 waves in both supraorbital stimulations (Fig. 1B). Visual evoked potentials exhibited poor wave formation on the right side. NCSs of all four limbs revealed delayed terminal latency, markedly slowed conduction velocity, and conduction block. These findings did not differ markedly from those of several previous NCSs performed serially over the previous 2 years.
Brain magnetic resonance imaging (MRI) showed fluid-attenuated inversion recovery (FLAIR) hyperintense signal changes with gadolinium contrast enhancement in the right optic nerve (Fig. 1C) and bilateral trigeminal nerves (Fig. 1D, E). Physical enlargement and increased FLAIR signal intensities of the right glossopharyngeal, vagus, and accessory nerves were also observed (Fig. 1F).
In cerebrospinal fluid (CSF) analysis, protein was mildly increased at 53.7 mg/dL and white blood cells were not detected. Assays for ganglioside antibodies including immunoglobulin (Ig) G and IgM antibodies against GM1, GM2, GD1a, GD1b, GT1a, GT1b, GQ1b, and GD3 were all negative. No specific abnormal finding was observed in the common blood count, hemoglobin A1C, blood chemistry, urine analysis, or serology and virology tests. Serum IgG antibody tests against aquaporin-4 antibody and myelin oligodendrocyte glycoprotein were negative, as were CSF oligoclonal band screening. PMP22, GJB1, and next-generation sequencing gene analyses for diagnosing Charcot-Marie-Tooth (CMT) disease were also negative.
The patient was diagnosed with classic CIDP involving bilateral facial and trigeminal nerves, right lower cranial nerves, and the right optic nerve. Steroid pulse therapy with methylprednisolone at 1 g/day for 5 days was administered, and the oral steroid was tapered while the immunosuppressant was maintained. The symptoms and signs for bilateral trigeminal neuropathy improved without any minimal neurological deficits, but decreased visual acuity in the right eye remained unchanged at the 6-month follow-up.
This case presented with typical CIDP accompanied by multiple cranial neuropathies involving the right optic and lower cranial nerves, and bilateral trigeminal and facial nerves. NCSs revealed a prolonged latency exceeding the upper normal limit by 50%, a decreased conduction velocity of more than 30% below the lower normal limit, a partial conduction block with the amplitude of the proximal negative peak CMAP being ≥ 50% lower than the distal negative peak, and no F-wave formation in at least two nerves.
The case did not present with ocular pain or papilledema despite the visual acuity, color test, visual field test, and visual evoked potential being abnormal on the right side. These findings make it likely that the patient had retrobulbar optic neuritis, which is compatible with the orbital MRI findings.
Both case reports and series of optic neuritis, transverse myelitis, multiple sclerosis, and other CNS lesions have been reported for patients with CIDP.4,5 Autoimmune disease that affects both the CNS and peripheral nervous system (PNS) has recently been proposed as ‘combined central and peripheral demyelination (CCPD)’.6 Even though the definition and pathophysiology of CCPD are still obscure, it can be hypothesized that this disease arises from an immunological attack simultaneously in the CNS and PNS.
Subclinical CNS demyelinating lesions are reportedly common in patients with anti-neurofascin-155-antibody-positive CIDP.7 The neurofascin-155 antibody test, which unfortunately was not available in our laboratory, can be helpful for elucidating CNS involvement in patients with CIDP, even though the present case did not have the typical symptoms or signs of neurofascin-155-antibody-related CIDP such as tremor, ataxia, and nystagmus. CNS involvement has also rarely been reported in patients with CMT disease showing clinical, electrophysiological, and radiological features similar to those of CIDP. The present case is likely to be closer to CIDP rather than to CMT due to the negative genetic tests (e.g., PMP22, GJB1, and next-generation sequencing gene analyses for diagnosing CMT), no family history of CMT, and good response to a steroid and immunosuppressant.
Bilateral trigeminal neuritis and right optic neuritis were symptomatic, while bilateral facial and right lower cranial neuropathies were asymptomatic. These cranial neuropathies are objectively elucidated through electrophysiology and MRI. It was particularly interesting that facial NCSs revealed bilateral facial neuropathies despite the patient not complaining of bifacial weakness. It was also very interesting to find physical enlargement and increased FLAIR signal intensities of the right glossopharyngeal, vagus, and accessory nerves in addition to in the right optic nerve and bilateral trigeminal nerves in brain MRI. Moreover, the case also did not present with the typical symptoms and signs of right lower cranial neuropathies, including tongue atrophy and deviation, dysarthria, dysphagia, and weakness of the trapezius and sternocleidomastoid muscles.
Cranial nerve involvement including ophthalmoplegia, facial weakness, facial paresthesia, dysarthria, and dysphagia are known to be as uncommon in CIDP as in Guillain-Barre syndrome. In particular, CNS involvement including optic neuritis has rarely been reported in CIDP.
The present case of CIDP suggests that asymptomatic cranial neuropathies in patients with CIDP have been underestimated in previous epidemiological studies. The findings of further epidemiological studies using brain MRI might more accurately reflect the frequency of cranial nerve involvement in patients with CIDP.
REFERENCES
1. Dyck PJ, O’Brien PC, Oviatt KF, Dinapoli RP, Daube JR, Bartleson JD, et al. Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Ann Neurol. 1982; 11:136–141.
2. Waddy HM, Misra VP, King RH, Thomas PK, Middleton L, Ormerod IE. Focal cranial nerve involvement in chronic inflammatory demyelinating polyneuropathy: clinical and MRI evidence of peripheral and central lesions. J Neurol. 1989; 236:400–405.
3. Holtkamp M, Zschenderlein R, Brück W, Weber JR. Chronic inflammatory demyelinating polyradiculoneuropathy with histologically proven optic neuritis. Acta Neuropathol. 2001; 101:529–531.
4. Sharma KR, Saadia D, Facca AG, Bhatia R, Ayyar DR, Sheremata W. Chronic inflammatory demyelinating polyradiculoneuropathy associated with multiple sclerosis. J Clin Neuromuscul Dis. 2008; 9:385–396.
5. Sarlani E, Grace EG, Balciunas BA, Schwartz AH. Trigeminal neuralgia in a patient with multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J Am Dent Assoc. 2005; 136:469–476.
6. Katchanov J, Lünemann JD, Masuhr F, Becker D, Ahmadi M, Bösel J, et al. Acute combined central and peripheral inflammatory demyelination. J Neurol Neurosurg Psychiatry. 2004; 75:1784–1786.
7. Kawamura N, Yamasaki R, Yonekawa T, Matsushita T, Kusunoki S, Nagayama S, et al. Anti-neurofascin antibody in patients with combined central and peripheral demyelination. Neurology. 2013; 81:714–722.
Fig. 1.
Findings of electrophysiological studies and brain magnetic resonance imaging (MRI) of the case. (A) Motor nerve conduction studies of both facial nerves showed delayed terminal latency on the right side and decreased compound muscle action potential amplitudes on both sides. (B) The blink reflex test showed delayed latencies of ipsilateral R1 and R2 waves and contralateral R2 waves in both supraorbital stimulations. Axial T1-weighted postcontrast brain MRI showed signal enhancement in the (C) right optic nerve (arrow), (D) right trigeminal nerve (arrow), and (E) cisternal segments of the left trigeminal nerve (arrow). (F) Coronal T2-weighted fluid-attenuated inversion recovery brain MRI showed asymmetric physical enlargement and increased signal intensities of the right glossopharyngeal nerve, vagus nerve, and spinal accessory nerve complex (arrow).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download