CASE
Fig. 1.
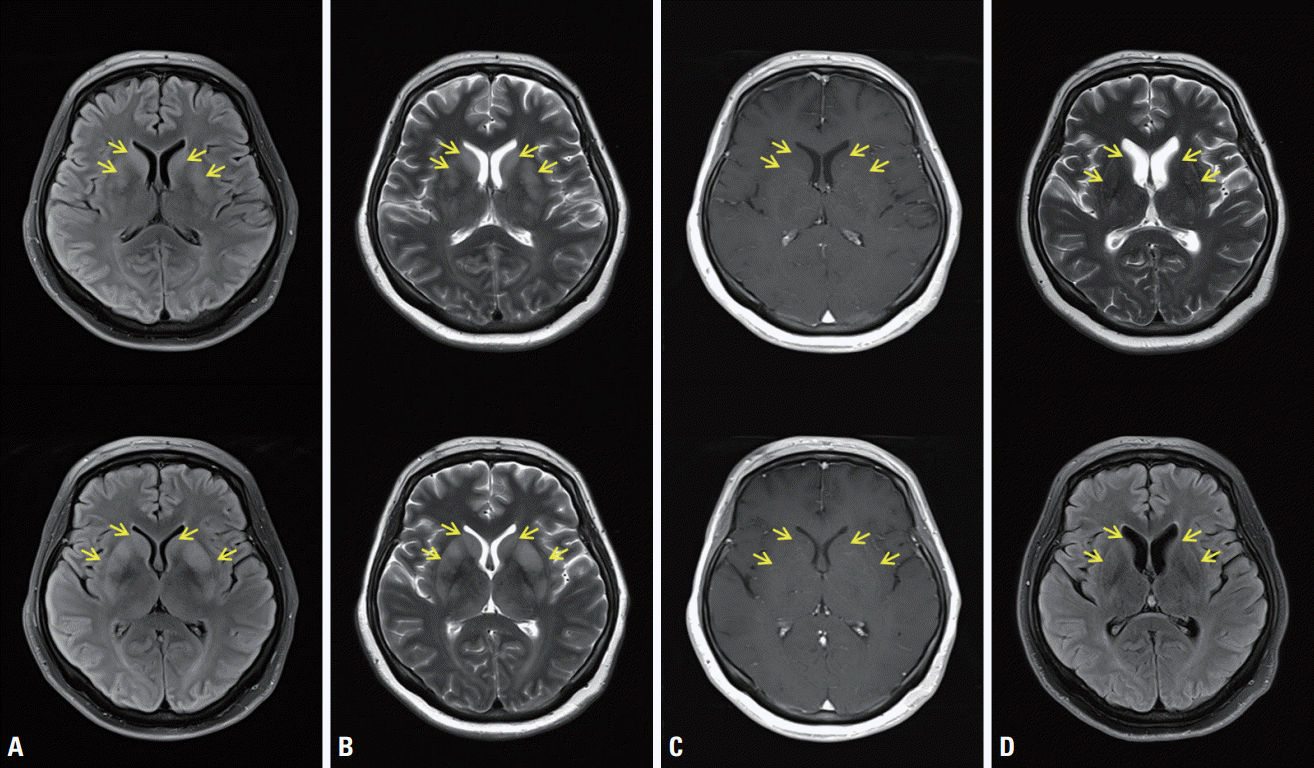
Fig. 2.
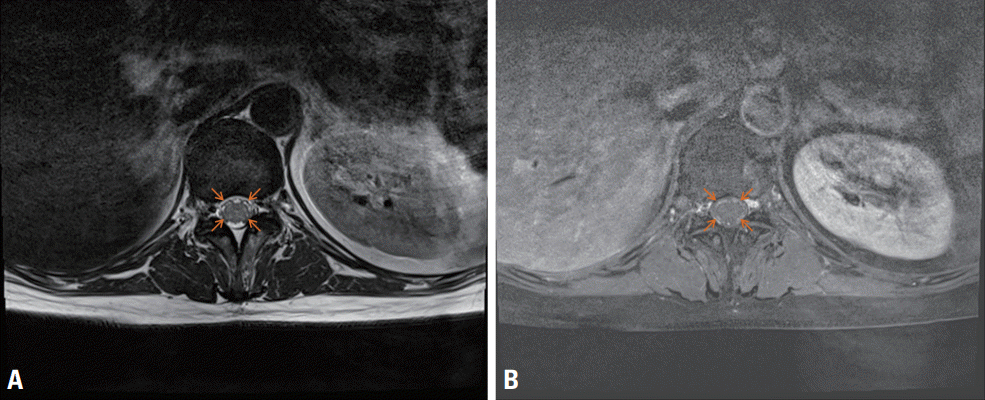
Table 1.
| Nerve |
Day 7 |
Day 21 |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
DL (ms) |
Amplitude (mV or μV) |
CV (m/s) |
F-wave |
H-reflex |
DL (ms) |
Amplitude (mV or μV) |
CV (m/s) |
F-wave |
H-reflex |
||||||||||||
| RT | LT | RT | LT | RT | LT | RT | LT | RT | LT | RT | LT | RT | LT | RT | LT | RT | LT | RT | LT | ||
| Median (M) | |||||||||||||||||||||
| Wrist | 2.6 | 2.5 | 11.6 | 8.1 | 24.9 | 24.0 | 2.6 | 2.5 | 9.0 | 6.8 | 26.1 | 26.1 | |||||||||
| Elbow | 11.6 | 7.9 | 61.8 | 62.3 | 8.3 | 6.7 | 60.7 | 58.7 | |||||||||||||
| Axilla | 10.1 | 6.3 | 61.5 | 65.8 | 7.9 | 6.7 | 62.3 | 63.2 | |||||||||||||
| Ref. | < 3.9 | > 5 | > 50 | < 26.5 | < 3.9 | > 5 | > 50 | < 26.5 | |||||||||||||
| Ulnar (M) | |||||||||||||||||||||
| Wrist | 1.9 | 2.0 | 10.0 | 9.8 | 24.8 | 24.9 | 2.1 | 2.0 | 6.3 | 7.5 | 25.8 | 27.2 | |||||||||
| BE | 10.0 | 9.8 | 63.1 | 64.3 | 6.3 | 7.4 | 59.6 | 62.0 | |||||||||||||
| AE | 9.5 | 9.6 | 61.5 | 44.9a | 6.2 | 5.8 | 58.5 | 34.3a | |||||||||||||
| Axilla | 8.8 | 9.3 | 64.9 | 64.9 | 6.1 | 5.5 | 55.8 | 53.9 | |||||||||||||
| Ref. | < 3.1 | > 6 | > 49 | < 27.3 | < 3.1 | > 6 | > 49 | < 27.3 | |||||||||||||
| Peroneal (M) | |||||||||||||||||||||
| Ankle | 3.8 | 3.3 | 3.2 | 3.8 | 41.0 | 42.4 | 3.9 | 3.5 | 1.1b | 0.6a,b | 47.4 | 45.5 | |||||||||
| FH | 3.1 | 3.7 | 53.7 | 53.0 | 1.1 | 0.6a | 47.8 | 46.5 | |||||||||||||
| Knee | 3.1 | 3.4 | 52.7 | 56.5 | 1.1 | 0.6a | 46.6 | 65.8 | |||||||||||||
| Ref. | < 5.5 | > 1 | > 40 | < 48.3 | < 5.5 | > 1 | > 40 | < 48.3 | |||||||||||||
| Tibial (M) | |||||||||||||||||||||
| Ankle | 2.9 | 3.3 | 16.9 | 19.3 | 40.1 | 41.7 | NRa | NRa | 3.3 | 4.0 | 7.2b | 6.4b | 47.6 | 46.4 | NRa | NRa | |||||
| Knee | 12.4 | 16.0 | 54.2 | 55.3 | 6.0 | 6.0 | 52.3 | 51.7 | |||||||||||||
| Ref. | < 5.6 | > 5 | > 41 | < 46 | < 28.5 | < 5.6 | > 5 | > 41 | < 46 | < 28.5 | |||||||||||
The first NCSs performed on day 7 after symptom onset are normal except mildly slowed left ulnar motor nerve conduction velocity (CV) and absent H-reflex bilaterally, suggestive of early Guillain-Barré syndrome. Follow-up NCSs performed on day 21 (2 weeks after 1st NCSs reveal markedly reduced compound muscle action potential (CMAP) in both peroneal and both tibial nerves and persistently absent H-reflex. Mildly reduced CMAP in both median and both ulnar nerves are also observed. In contrast, results of sensory NCSs are completely normal in successive two tests (Supplementary Table 1). No parameters exceeding demyelinating criteria are observed such as prolonged motor distal latency 150% above upper limit of normal, slowing CV 70% less than lower limit of normal, motor conduction block or/and abnormal temporal dispersion. Taken together, these results are compatible to acute motor axonal neuropathy.
DL, distal latency; CV, conduction velocity; RT, right; LT, left; M, motor; Ref., reference range; BE, below the elbow; AE, above the elbow; FH, fibula head; NR, no response.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download