1. Fabris E, Kilic S, Schellings DAAM, et al. Long-term mortality and prehospital tirofiban treatment in patients with ST elevation myocardial infarction. Heart. 2017; 103:1515–1520. PMID:
28679686.
2. Hinton J, Mariathas M, Gabara L, et al. Distribution of contemporary sensitivity troponin in the emergency department and relationship to 30-day mortality: The CHARIOT-ED substudy. Clin Med (Lond). 2020; 20:528–534. PMID:
33199315.
3. Menees DS, Peterson ED, Wang Y, et al. Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013; 369:901–909. PMID:
24004117.
4. Olier I, Sirker A, Hildick-Smith DJR, et al. Association of different antiplatelet therapies with mortality after primary percutaneous coronary intervention. Heart. 2018; 104:1683–1690. PMID:
29437885.
5. Gong X, Ding X, Chen H, Li H. Real-world use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers/β-blocks in Chinese patients before acute myocardial infarction occurs: patient characteristics and hospital follow-up. J Transl Med. 2018; 16:346. PMID:
30526628.
6. Maslov LN, Barbarash OL. Pharmacological approaches to limiting the infarct zone size in patients with acute myocardial infarction: analysis of clinical data. Eksp Klin Farmakol. 2018; 81:34–41.
7. Ndrepepa G. Improving myocardial injury, infarct size, and myocardial salvage in the era of primary PCI for STEMI. Coron Artery Dis. 2015; 26:341–355. PMID:
25715338.
8. Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol. 2015; 78:23–34. PMID:
25446182.
9. Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977; 56:786–794. PMID:
912839.
10. Cohen MV, Downey JM. Combined cardioprotectant and antithrombotic actions of platelet P2Y12 receptor antagonists in acute coronary syndrome: just what the doctor ordered. J Cardiovasc Pharmacol Ther. 2014; 19:179–190. PMID:
24298192.
11. Cohen MV, Downey JM. The impact of irreproducibility and competing protection from P2Y
12 antagonists on the discovery of cardioprotective interventions. Basic Res Cardiol. 2017; 112:64. PMID:
28952016.
12. Mirabet M, Garcia-Dorado D, Inserte J, et al. Platelets activated by transient coronary occlusion exacerbate ischemia-reperfusion injury in rat hearts. Am J Physiol Heart Circ Physiol. 2002; 283:H1134–H1141. PMID:
12181144.
13. Ault KA, Cannon CP, Mitchell J, et al. Platelet activation in patients after an acute coronary syndrome: results from the TIMI-12 trial. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1999; 33:634–639. PMID:
10080462.
14. Langford EJ, Wainwright RJ, Martin JF. Platelet activation in acute myocardial infarction and unstable angina is inhibited by nitric oxide donors. Arterioscler Thromb Vasc Biol. 1996; 16:51–55. PMID:
8548426.
15. Russo I, Femminò S, Barale C, et al. Cardioprotective properties of human platelets are lost in uncontrolled diabetes mellitus: a study in isolated rat hearts. Front Physiol. 2018; 9:875. PMID:
30042694.
16. Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015; 116:674–699. PMID:
25677517.
17. Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020; 17:773–789. PMID:
32620851.
18. Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003; 83:1113–1151. PMID:
14506302.
19. Yang XM, Liu Y, Cui L, et al. Platelet P2Y
12 blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther. 2013; 18:251–262. PMID:
23233653.
20. Birnbaum Y, Lin Y, Ye Y, et al. Aspirin before reperfusion blunts the infarct size limiting effect of atorvastatin. Am J Physiol Heart Circ Physiol. 2007; 292:H2891–H2897. PMID:
17277020.
21. Gross ER, Hsu AK, Gross GJ. Acute aspirin treatment abolishes, whereas acute ibuprofen treatment enhances morphine-induced cardioprotection: role of 12-lipoxygenase. J Pharmacol Exp Ther. 2004; 310:185–191. PMID:
14993258.
22. Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan MK, Nylander S, Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2015; 35:1805–1814. PMID:
26044583.
23. Cattaneo M. P2Y
12 receptors: structure and function. J Thromb Haemost. 2015; 13(Suppl 1):S10–S16. PMID:
26149010.
24. Djerada Z, Feliu C, Richard V, Millart H. Current knowledge on the role of P2Y receptors in cardioprotection against ischemia-reperfusion. Pharmacol Res. 2017; 118:5–18. PMID:
27520402.
25. Cattaneo M. New P2Y
12 inhibitors. Circulation. 2010; 121:171–179. PMID:
20048234.
26. Barrabés JA, Inserte J, Mirabet M, et al. Antagonism of P2Y
12 or GPIIb/IIIa receptors reduces platelet-mediated myocardial injury after ischaemia and reperfusion in isolated rat hearts. Thromb Haemost. 2010; 104:128–135. PMID:
20431845.
27. Yang XM, Cui L, Alhammouri A, Downey JM, Cohen MV. Triple therapy greatly increases myocardial salvage during ischemia/reperfusion in the in situ rat heart. Cardiovasc Drugs Ther. 2013; 27:403–412. PMID:
23832692.
28. Audia JP, Yang XM, Crockett ES, et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y
12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol. 2018; 113:32. PMID:
29992382.
29. Liu X, Gu Y, Liu Y, Zhang M, Wang Y, Hu L. Ticagrelor attenuates myocardial ischaemia-reperfusion injury possibly through downregulating galectin-3 expression in the infarct area of rats. Br J Clin Pharmacol. 2018; 84:1180–1186. PMID:
29381821.
30. Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014; 19:209–219. PMID:
24414167.
31. Yang XM, Gadde S, Audia JP, Alvarez DF, Downey JM, Cohen MV. Ticagrelor does not protect isolated rat hearts, thus clouding its proposed cardioprotective role through ENT 1 in heart tissue. J Cardiovasc Pharmacol Ther. 2019; 24:371–376. PMID:
30744423.
32. Yang XM, Liu Y, Cui L, et al. Two classes of anti-platelet drugs reduce anatomical infarct size in monkey hearts. Cardiovasc Drugs Ther. 2013; 27:109–115. PMID:
23318690.
33. Liu X, Wang Y, Zhang M, Liu Y, Hu L, Gu Y. Ticagrelor reduces ischemia-reperfusion injury through the NF-κB-dependent pathway in rats. J Cardiovasc Pharmacol. 2019; 74:13–19. PMID:
31274838.
34. Maslov LN, Popov SV, Mukhomedzyanov AV, et al. Reperfusion cardiac injury: receptors and the signaling mechanisms. Curr Cardiol Rev. 2022.
35. Vilahur G, Gutiérrez M, Casani L, et al. P2Y
12 antagonists and cardiac repair post-myocardial infarction: global and regional heart function analysis and molecular assessments in pigs. Cardiovasc Res. 2018; 114:1860–1870. PMID:
30124783.
36. Birnbaum Y, Tran D, Chen H, Nylander S, Sampaio LC, Ye Y. Ticagrelor improves remodeling, reduces apoptosis, inflammation and fibrosis and increases the number of progenitor stem cells after myocardial infarction in a rat model of ischemia reperfusion. Cell Physiol Biochem. 2019; 53:961–981. PMID:
31820856.
37. Xie ZJ, Xin SL, Chang C, et al. Combined glycoprotein IIb/IIIa inhibitor therapy with ticagrelor for patients with acute coronary syndrome. PLoS One. 2021; 16:e0246166. PMID:
33529262.
38. Yang XM, Downey JM, Cohen MV, Housley NA, Alvarez DF, Audia JP. The highly selective caspase-1 inhibitor VX-765 provides additive protection against myocardial infarction in rat hearts when combined with a platelet inhibitor. J Cardiovasc Pharmacol Ther. 2017; 22:574–578. PMID:
28399648.
39. Hjortbak MV, Olesen KKW, Seefeldt JM, et al. Translation of experimental cardioprotective capability of P2Y
12 inhibitors into clinical outcome in patients with ST-elevation myocardial infarction. Basic Res Cardiol. 2021; 116:36. PMID:
34037861.
40. Penna C, Aragno M, Cento AS, et al. Ticagrelor conditioning effects are not additive to cardioprotection induced by direct NLRP3 inflammasome inhibition: role of RISK, NLRP3, and redox cascades. Oxid Med Cell Longev. 2020; 2020:9219825. PMID:
32832010.
41. Cohen MV, Yang XM, White J, Yellon DM, Bell RM, Downey JM. Cangrelor-mediated cardioprotection requires platelets and sphingosine phosphorylation. Cardiovasc Drugs Ther. 2016; 30:229–232. PMID:
26780906.
42. Ahmed N, Linardi D, Muhammad N, et al. Sphingosine 1-phosphate receptor modulator fingolimod (FTY720) attenuates myocardial fibrosis in post-heterotopic heart transplantation. Front Pharmacol. 2017; 8:645. PMID:
28966593.
43. Chen R, Cai X, Liu J, Bai B, Li X. Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis. Life Sci. 2018; 215:31–42. PMID:
30367841.
44. Knapp M. Cardioprotective role of sphingosine-1-phosphate. J Physiol Pharmacol. 2011; 62:601–607. PMID:
22314562.
45. Jin ZQ, Zhou HZ, Zhu P, et al. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002; 282:H1970–H1977. PMID:
12003800.
46. Means CK, Xiao CY, Li Z, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007; 292:H2944–H2951. PMID:
17293497.
47. Vessey DA, Li L, Kelley M, Karliner JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008; 375:425–429. PMID:
18706887.
48. Hofmann U, Burkard N, Vogt C, et al. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc Res. 2009; 83:285–293. PMID:
19416991.
49. Marino A, Sakamoto T, Robador PA, Tomita K, Levi R. S1P receptor 1-mediated anti-renin-angiotensin system cardioprotection: pivotal role of mast cell aldehyde dehydrogenase type 2. J Pharmacol Exp Ther. 2017; 362:230–242. PMID:
28500264.
50. Kuai F, Wang L, Su J, Wang Y, Han Y, Zhou S. Exploring the protective role and the mechanism of sphingosine 1 phosphate in endotoxic cardiomyocytes. Shock. 2019; 52:468–476. PMID:
30300318.
51. Spampinato SF, Sortino MA, Salomone S. Sphingosine-1-phosphate and sphingosine-1-phosphate receptors in the cardiovascular system: pharmacology and clinical implications. Adv Pharmacol. 2022; 94:95–139. PMID:
35659378.
52. Kelly-Laubscher RF, King JC, Hacking D, et al. Cardiac preconditioning with sphingosine-1-phosphate requires activation of signal transducer and activator of transcription-3. Cardiovasc J S Afr. 2014; 25:118–123.
53. Fang R, Zhang LL, Zhang LZ, Li W, Li M, Wen K. Sphingosine 1-phosphate postconditioning protects against myocardial ischemia/reperfusion injury in rats via mitochondrial signaling and Akt-Gsk3β phosphorylation. Arch Med Res. 2017; 48:147–155. PMID:
28625317.
54. Chawla S, Rahar B, Saxena S. S1P prophylaxis mitigates acute hypobaric hypoxia-induced molecular, biochemical, and metabolic disturbances: a preclinical report. IUBMB Life. 2016; 68:365–375. PMID:
26959531.
55. Rutherford C, Childs S, Ohotski J, et al. Regulation of cell survival by sphingosine-1-phosphate receptor S1P1 via reciprocal ERK-dependent suppression of Bim and PI-3-kinase/protein kinase C-mediated upregulation of Mcl-1. Cell Death Dis. 2013; 4:e927. PMID:
24263101.
56. Somers SJ, Frias M, Lacerda L, Opie LH, Lecour S. Interplay between SAFE and RISK pathways in sphingosine-1-phosphate-induced cardioprotection. Cardiovasc Drugs Ther. 2012; 26:227–237. PMID:
22392184.
57. Kuang Y, Li X, Liu X, et al. Vascular endothelial S1pr1 ameliorates adverse cardiac remodelling via stimulating reparative macrophage proliferation after myocardial infarction. Cardiovasc Res. 2021; 117:585–599. PMID:
32091582.
58. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001; 345:494–502. PMID:
11519503.
59. Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005; 366:1607–1621. PMID:
16271642.
60. Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA. 2005; 294:1224–1232. PMID:
16143698.
61. Lewis BS, Mehta SR, Fox KA, et al. Benefit of clopidogrel according to timing of percutaneous coronary intervention in patients with acute coronary syndromes: further results from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Am Heart J. 2005; 150:1177–1184. PMID:
16338255.
62. Dangas G, Mehran R, Guagliumi G, et al. Role of clopidogrel loading dose in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: results from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. J Am Coll Cardiol. 2009; 54:1438–1446. PMID:
19796737.
63. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361:1045–1057. PMID:
19717846.
64. Witkowski A, Maciejewski P, Wasek W, et al. Influence of different antiplatelet treatment regimens for primary percutaneous coronary intervention on all-cause mortality. Eur Heart J. 2009; 30:1736–1743. PMID:
19376786.
65. Song YB, Hahn JY, Gwon HC, et al. A high loading dose of clopidogrel reduces myocardial infarct size in patients undergoing primary percutaneous coronary intervention: a magnetic resonance imaging study. Am Heart J. 2012; 163:500–507. PMID:
22424023.
66. Leonardi S, Stebbins A, Lopes RD, et al. Quantification of the effect of clopidogrel on enzymatic infarct size related to a percutaneous coronary intervention in patients with acute coronary syndromes: insights from the CHAMPION percutaneous coronary intervention trial. Coron Artery Dis. 2013; 24:321–327. PMID:
23442944.
67. Serebruany VL, Cherepanov V, Tomek A, Kim MH. Among antithrombotic agents, prasugrel, but not ticagrelor, is associated with reduced 30 day mortality in patients with ST-elevated myocardial infarction. Int J Cardiol. 2015; 195:104–110. PMID:
26043353.
68. Kang HJ, Clare RM, Gao R, et al. Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: a retrospective analysis from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Am Heart J. 2015; 169:899–905.e1. PMID:
26027629.
69. Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome -- randomized, double-blind, phase III PHILO study. Circ J. 2015; 79:2452–2460. PMID:
26376600.
70. Khan JN, Greenwood JP, Nazir SA, et al. Infarct size following treatment with second- versus third-generation P2Y
12 antagonists in patients with multivessel coronary disease at ST-segment elevation myocardial infarction in the CvLPRIT study. J Am Heart Assoc. 2016; 5:e003403. PMID:
27247336.
71. Yun KH, Rhee SJ, Ko JS. Comparison of the infarct size between the loading of ticagrelor and clopidogrel in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Korean Circ J. 2017; 47:705–713. PMID:
28955389.
72. Huber K, Ducrocq G, Hamm CW, et al. Early clinical outcomes as a function of use of newer oral P2Y
12 inhibitors versus clopidogrel in the EUROMAX trial. Open Heart. 2017; 4:e000677. PMID:
29225903.
73. Kang J, Han JK, Ahn Y, et al. Third-generation P2Y
12 inhibitors in East Asian acute myocardial infarction patients: a nationwide prospective multicentre study. Thromb Haemost. 2018; 118:591–600. PMID:
29534250.
74. Gao R, Wu Y, Liu H, et al. Safety and incidence of cardiovascular events in chinese patients with acute coronary syndrome treated with ticagrelor: the 12-month, phase IV, multicenter, single-arm DAYU study. Cardiovasc Drugs Ther. 2018; 32:47–56. PMID:
29488142.
75. Ubaid S, Ford TJ, Berry C, et al. Cangrelor versus ticagrelor in patients treated with primary percutaneous coronary intervention: impact on platelet activity, myocardial microvascular function and infarct size: a randomized controlled trial. Thromb Haemost. 2019; 119:1171–1181. PMID:
31129911.
76. Dworeck C, Redfors B, Angerås O, et al. Association of pretreatment with P2Y
12 receptor antagonists preceding percutaneous coronary intervention in non-ST-segment elevation acute coronary syndromes with outcomes. JAMA Netw Open. 2020; 3:e2018735. PMID:
33001202.
77. Moura Guedes JP, Marques N, Azevedo P, et al. P2Y
12 inhibitor loading dose before catheterization in ST-segment elevation myocardial infarction: is this the best strategy? Rev Port Cardiol (Engl Ed). 2020; 39:553–561. PMID:
33023777.
78. Al Raisi S, Protty M, Raposeiras-Roubín S, et al. Ticagrelor versus prasugrel in acute coronary syndrome: sex-specific analysis from the RENAMI registry. Minerva Cardiol Angiol. 2021; 69:408–416. PMID:
34137238.
79. Park Y, Koh JS, Lee JH, et al. Effect of ticagrelor on left ventricular remodeling in patients with ST-segment elevation myocardial infarction (HEALING-AMI). JACC Cardiovasc Interv. 2020; 13:2220–2234. PMID:
33032710.
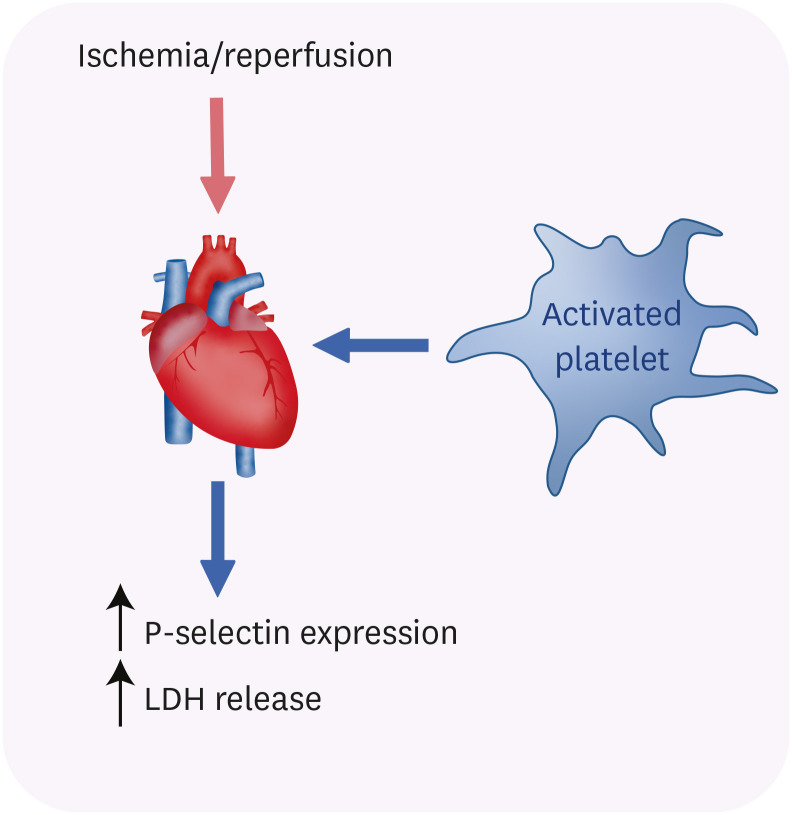
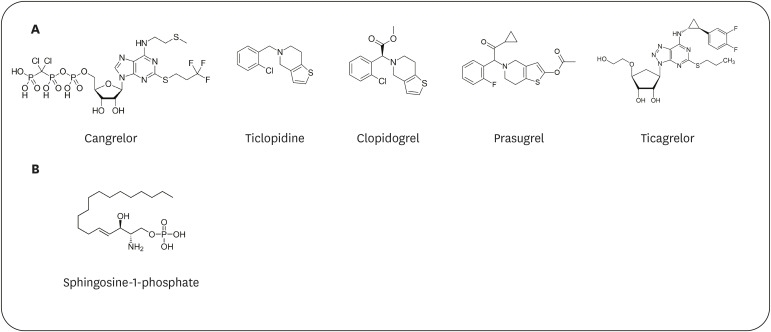
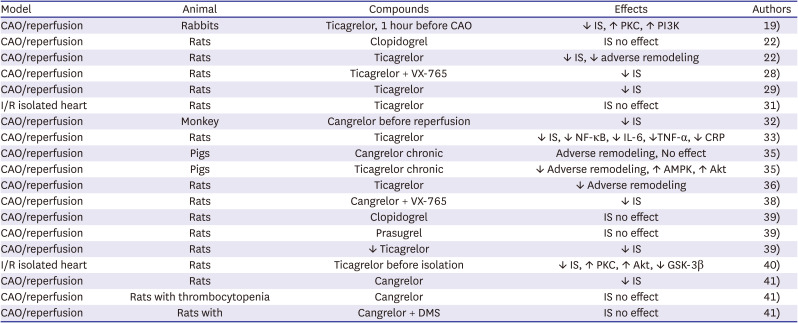
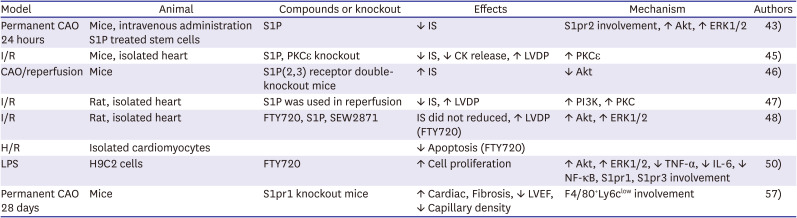




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download