INTRODUCTION
Catheter ablation (CA) is a well-established therapy for the treatment of atrial fibrillation (AF), and CA is superior to anti-arrhythmic drugs (AADs) in preventing AF recurrences and alleviating AF-related symptoms.
1) Pulmonary vein isolation (PVI) is the cornerstone strategy of CA in the management of patients with either paroxysmal AF (PAF) or persistent AF (PsAF).
2) In addition to radiofrequency ablation (RFA), cryoballoon ablation (CBA) with the Arctic Front Advance catheter has become a “gold standard” for AF ablation since 2012 and has more recently gained global acceptance.
3) CBA-PVI has several distinct advantages over RFA, including shorter procedures, less operator-dependent experience for usage, and a shorter learning curve to establish mastery.
4)5)6) Recently, 2 (single-center) trials have shown the efficacy and safety of Arctic Front Advance CBA for the treatment of patients with PAF and PsAF in Korea
7)8); however, to our knowledge, the current analysis is the first multicenter study describing the outcomes of CBA in real-world Korea clinical practice.
Go to :

METHODS
Ethical statement
Data collection adhered to the principles outlined in the Declaration of Helsinki (2013) and Good Clinical Practices. The study was approved by local institutional review boards (IRBs) and ethics committees at each participating center, and patients provided written informed consent prior to participation in the registry (Samsung Medical Center IRB 2019-02-061, Seoul National University Bundang Hospital B-1903/562-303 and Hallym University Sacred Heart Hospital 2019-04-025). The data, analytic methods, and study materials will not be available to other researchers. Specifically, patient data privacy within the Cryo Global Registry does not allow nor consent to data sharing with outside parties.
Study design
The Cryo Global Registry (
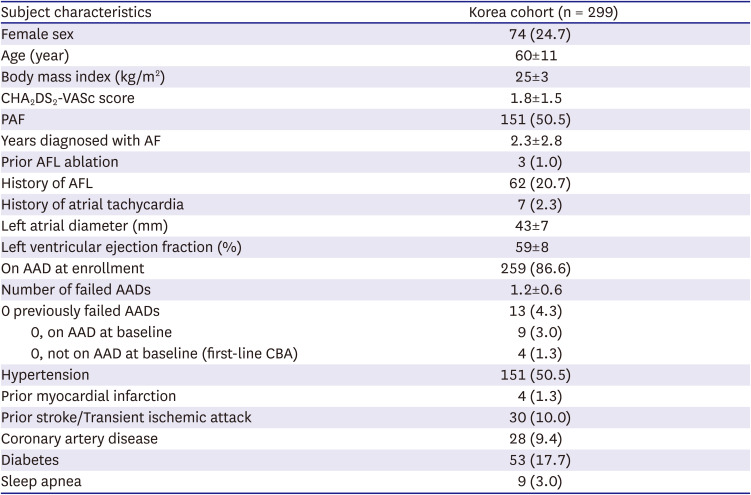
NCT02752737) is an ongoing, prospective, multicenter post-market registry to evaluate outcomes on AF ablation procedures performed with the Arctic Front Advance Family of cryoablation catheters (Medtronic, Inc., Minneapolis, MN, USA). The current analyses aimed to describe patient baseline characteristics, procedural characteristics, and outcomes of CBA in the Korean sub-population within the Cryo Global Registry. Data regarding the ablation procedures were collected from 299 patients enrolled in 3 hospitals in Korea.
The registry has been previously described in detail by Chun et al.
9) In brief, a global steering committee of international physicians oversees data quality, analyses, and publication milestones. Procedures performed in this study were performed according to standard-of-care at the time of data collection.
Patient population
Patients in this analysis were consecutively enrolled between April 2019 to May 2020 at 3 hospitals in Korea. All patients ≥18 years old with a planned CBA procedure were eligible for inclusion in the registry and were not excluded based on pre-existing characteristics nor medical conditions. All patients received PVI ablation for the first time, and patients were classified by AF disease status, including: PAF (AF that terminates spontaneously or with intervention within 7 days of onset), PsAF (continuous AF that is sustained beyond 7 days and ≤12 months), or long-standing PsAF (LsPsAF, continuous AF >12 months).
3) LsPsAF patients were pooled with PsAF patients in this analysis of 3 centers for statistical considerations because of sample size considerations.
Cryoballoon ablation procedure
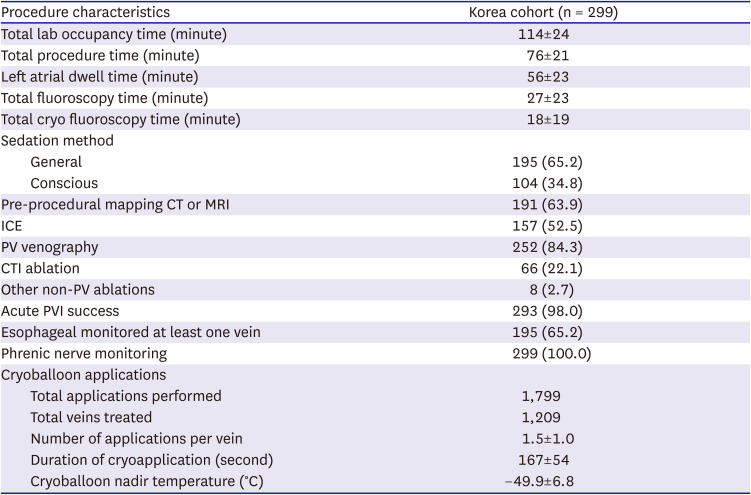
CBA-PVI was performed according to local standard-of-care in Korea and in accordance with typical procedural techniques.
7)8)9) In brief, a dedicated 15-F OD steerable sheath (FlexCath Advance Steerable Sheath; Medtronic, Inc.) was used to introduce a 23- or 28-mm CBA catheter (Arctic Front Advance; Medtronic, Inc.) into the left atrium (LA). The CBA catheter was maneuvered in the LA either over a J-tip guidewire or a dedicated inner-lumen (octopolar or decapolar) circular mapping catheter (Achieve or Achieve Advance; Medtronic, Inc.). The CBA catheter was inflated and advanced towards the antral surface of the pulmonary vein (PV). Freezing was initiated upon antral occlusion of the targeted PV. The number of cryoapplications and freeze duration were operator determined, and PVI was confirmed by entrance and/or exit block after CA.
Phrenic nerve monitoring (by diagnostic catheter pacing at the subclavian vein and diaphragmatic movement monitoring by manual palpitation and/or adjunctive diaphragmatic movement detection methods) was recommended during all right-sided PVI. Freezes were stopped upon detection of an attenuated diaphragmatic response. Pre- and intra-procedural imaging, esophageal temperature monitoring, and post-ablation chemical testing were determined by the operator. Additional ablation tools used (e.g., focal cryoablation and radiofrequency CA), as well as lesions adjunctive to PVI, were left to the discretion of the operator and were documented. Periprocedural anti-coagulation and AAD initiation/continuation were determined by the operator, and patients were discharged according to the hospital’s standard-of-care practice.
Patient follow-up and endpoints
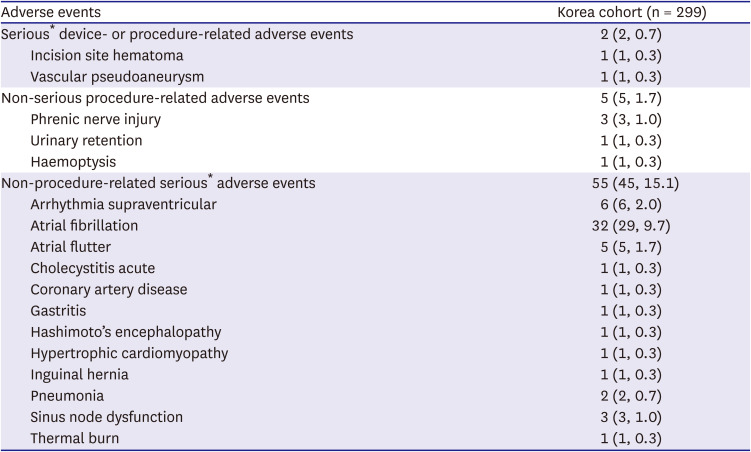
Patients were followed via telephone and/or in-office visits according to their hospital’s standard-of-care method. Patients were protocol-required to have at least one status visit at 12 months after the index CBA. The primary objective of the registry was to report efficacy and safety after the index CBA procedure throughout 12 months. The Korean patients that are represented in this analysis are a subset population from the larger Cryo Global AF Registry. Procedural efficacy was assessed by time-to-first ≥30-second recurrence of AF and atrial arrhythmias (defined as AF/atrial flutter [AFL]/atrial tachycardia [AT]) between a 90-day blanking period and 12-month follow-up. The method of rhythm monitoring was not dictated by the study protocol and could be conducted by any of the following methods, including: 12-lead electrocardiogram (ECG), Holter monitor, trans-telephonic monitor, insertable cardiac monitor, pacemaker, and/or implantable cardioverter defibrillator. The safety endpoint was the serious device and/or procedure-related adverse event rate as classified by the physician. Non-serious procedure-related adverse events and non-procedure related serious adverse events were also reported. Serious adverse events were defined according to international standards (ISO 14 155:2001) and included all events that led to death, or to a serious deterioration in health that resulted in either (a) a life-threatening illness or injury, (b) a permanent impairment in body structure or function, (c) in-patient or prolonged hospitalization, or (d) medical intervention to prevent life-threatening illness or injury.
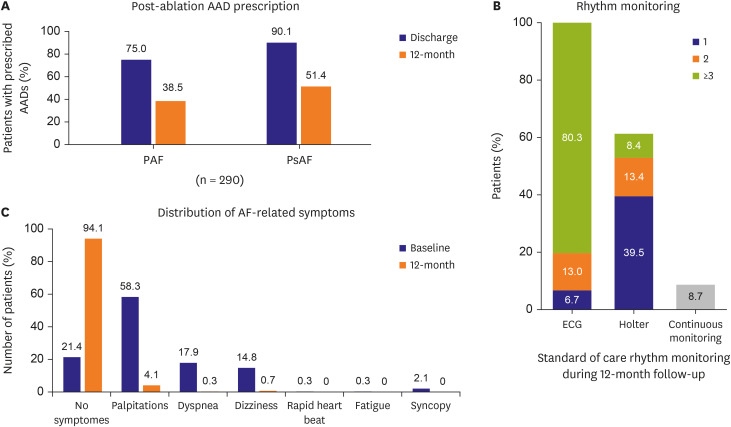
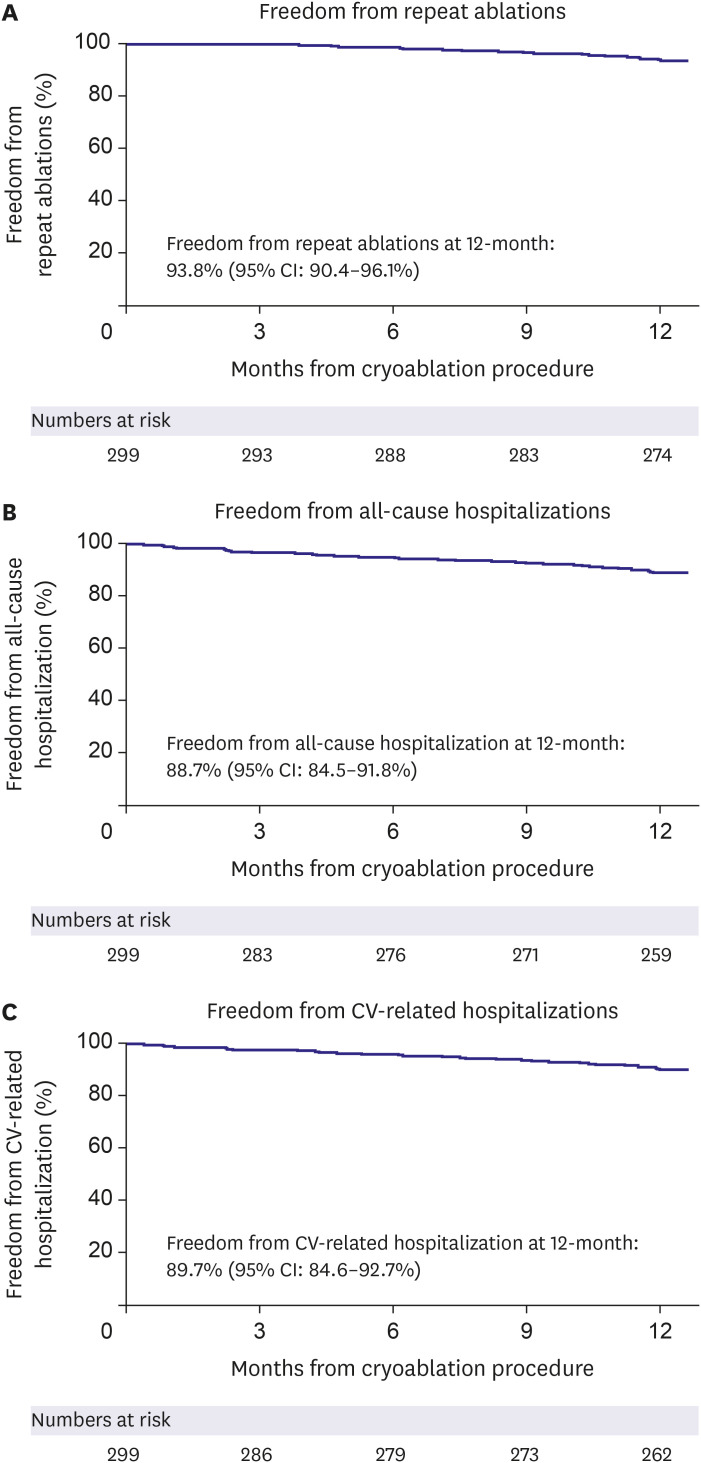
Ancillary objectives were examined in this analysis including baseline characteristics, procedural characteristics, quality of life (QoL), arrhythmia monitoring, post-ablation medication management, and hospitalization rates. QoL (measured by 3-level EuroQol 5-dimensional questionnaire [EQ-5D-3L]) and predefined arrhythmia symptoms were monitored at baseline and 12-month follow-up. The EQ-5D-3L questionnaire measures 5 dimensions of health, including: 1) mobility, 2) selfcare, 3) physical activities, 4) pain and discomfort, and 5) anxiety and depression. Each question has 3 levels of response indicating no problem, some problem, or extreme problem. The resulting index score is on a scale ranging from 0 (least healthy) to 1 (most healthy). Class I/III AAD utilization was assessed at baseline, after hospital discharge for post-ablation care, and at the 12-month visit. The method and frequency of rhythm monitoring was documented throughout 12 months of follow-up, as well as the all-cause and cardiovascular (CV)-related hospitalization rates, and the repeat ablation rate.
Statistical analysis
Baseline characteristics and clinical data were summarized using the appropriate summary statistics. Continuous variables were summarized as mean and standard deviation, and categorical variables were summarized as counts and percentages. The Kaplan-Meier method was used to estimate the 12-month freedom from AF recurrence, all atrial arrhythmia recurrence, repeat ablation, and rehospitalization. Standard error was calculated with Greenwood’s formula. Separate log-rank tests were performed to assess the rate of AF recurrence and all atrial arrhythmia recurrence between PAF and PsAF subjects. Changes in QoL was assessed with a one-sample t-test. Values of p<0.05 were considered statistically significant. Statistical analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Go to :

DISCUSSION
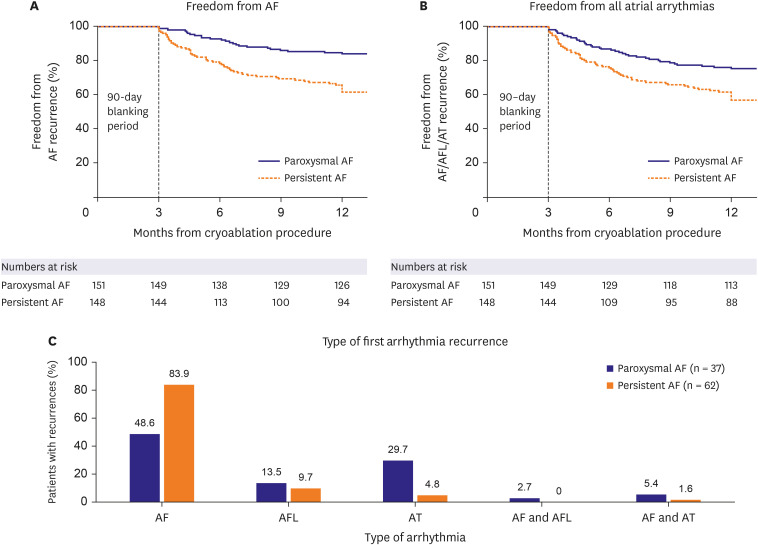
This sub-analysis of the Cryo Global Registry assessed the efficacy and safety of CBA using the Arctic Front Advance balloon for the treatment of patients with AF in Korea. In patients with PAF, freedom from a ≥30-second recurrence of AF was 83.9% at 12 months, and freedom from AF/AFL/AT was 75.2% at 12 months. Freedom from AF and AF/AFL/AT at 12 months was 61.6% and 56.7%, respectively, in the PsAF patient cohort. The rate of serious index procedure-related adverse events was 0.7%. Also, QoL significantly improved at 12-month post-ablation, and 94.1% of patients were free of AF-related symptoms at 12-month follow-up. Repeat ablations were performed in 6.2% of patients during the follow-up period, and 88.7% of patients were free from all-cause hospitalization at 12-month post-ablation.
Cappato et al.
7) was the first to report on real-world evidence of outcome of CA in AF on a global scale in 2005. While the novel real-world evidence was unique and useful, the method of collection was a survey with inherent limitations. More recently, several modern registries have followed and are still ongoing. These have proven to be useful by collecting global information on patient demographics and routine clinical practice (e.g., timing and type of treatment), analyzing trends in procedural techniques, monitoring overall safety, and identifying risk factors and predictors of outcomes in large populations with different co-morbidities.
8) The present analysis provides insight into the real-world use, safety and efficacy of CBA across multiple Korean centers within the larger Cryo Global Registry.
9) The outcome of this Korean real-world evidence is compared below to registries around the globe.
10)11)12)13) In addition, there are currently 2 publications on CBA-PVI in the Korean population for comparison of procedural outcome, and both are single-center experiences.
14)15)
Patient demographics of Korean patients in this sub-analysis of the Cryo Global Registry were very comparable to what was previously reported for age, echocardiogram parameters, co-morbidities, number of failed AADs, and time to ablation.
9)10)11)12)13) The participating Korean centers enrolled a slightly higher number of males (75.3%) compared to other registries. Half of the Korean patients had non-PAF, which was also seen in the GWTG-AFIB registry,
10) but is higher than reported in the global cohort of the Cryo Global Registry.
9) LAD was slightly larger than expected in the Korean sub-population (43±7 mm), when comparing to other registry populations. Also, 20.7% of Korean patients had a history of AFL, which is observed in some
11)12) but not all registry cohorts.
9)10)13)
Cryoballoon procedures were performed following current trends. Adjunctive pre- and peri-procedural imaging and monitoring were frequently used. CBA procedure duration in this multicenter analysis (76±21 minutes) was relatively short when compared to the 2 published studies from Korea and other real-world evidence (ranging from 73±26 minutes to 136.0±46.5 minutes)
9)10)11)12)13)14)15) especially when considering the number of ablations beyond PVI in this sub-analysis. A history of AFL at baseline was documented in 20.7% of patients, which explains the relatively high number of CTI ablations (22.1%) performed at the index procedure. Non-PVI ablation added on average 9 minutes to the total fluoroscopy time. The mean number of freeze applications per vein was 1.5±1.0, with a mean duration of 167±54 seconds per application, in line with contemporary dosing strategies for the Arctic Front Advance cryoballoon.
9)12)13)
Freedom from AF in paroxysmal patients was within range of what was previously reported.
9)12) Freedom from AF in the persistent population was significantly lower with 61.6% in the Korean sub-population vs. 73% reported by Chun et al.
9) and Ferrero-De-Loma-Osorio et al.
12) in the Cryo Global Registry and RECABA registry, respectively. In addition, the effectiveness of CBA for the prevention of all arrythmia recurrences (AF/AFL/AT) in the Korean sub-population was lower than expected based on outcome of the global and Japan cohorts of the Cryo Global Registry.
9)13) However, it should be noted that monitoring was more rigorously performed in the Korean sub-population. The main type of arrhythmia recurrence in the present analysis was AF in 83.0% of PsAF patients vs. 48.6% in PAF patients. There was a relatively high number of patients with an AT (29.7%) and AFL (13.5%) type of recurrence in the PAF cohort compared to the other 2 Korean trials and the overall Cryo Global Registry population.
9)14)15) This may be explained by the relatively high number of PAF patients enrolled in the registry with a history of AFL (22.5%) and AT (4.0%) at baseline.
AAD use was not dictated by the study protocol. According to standard of care in the participating centers, AAD were prescribed for post-ablation care in 82.4% of patients. A significant reduction in AAD use from baseline (86.6%) to 12 months (44.8%) was observed in the current analysis. Prescription of AAD at discharge and 12 months follow-up was notably higher in the 3 participating centers then in the global cohort and the Korean patients in the single-center trial described by Pak et al.
14)
The total rate of procedure-related events during standard-of-care in this multicenter registry was low overall (0.7% serious and 1.7% of non-serious events). These findings on low rates of complications following a CBA procedure are consistent with previously published literature, which demonstrates the consistent safety profile across real-world usage for CBA-PVI.
4)5)6)9)10)11)12)13)14) Importantly, in this analysis of 299 patients, no atrioesophageal fistula, pericardial tamponade, or PV stenosis were reported, while 12-month QoL improved significantly for the cohort.
There is limitation to our study. This sub-analysis of the Cryo Global Registry describes the clinical performance and safety of CBA with Arctic Front Advance according to real-world practice in Korea. Procedure, cardiac monitoring and AAD prescription post-ablation were not prescribed. Non-standardized monitoring could have led to under-detection of AF, especially asymptomatic AF recurrence.
15) Continuous monitoring was not available in most patients, and therefore, episodes of asymptomatic AF might not have been uniformly identified. However, the current analysis describes standard of care in the participating centers. Most patients (93.3%) had a monitoring event at least 3 times during the follow-up period, mainly 12-lead ECG and Holter monitoring. The current monitoring protocol was comparable to other real-world evidence publications.
9)11)12)
In conclusions, CBA according to standard-of-care usage in Korea is safe and effective in preventing AF recurrence, repeat ablation, CV-related hospitalization, and AF-related symptoms at 12 months after the index procedure.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download