1. Dunlay SM, Foxen JL, Cole T, et al. A survey of clinician attitudes and self-reported practices regarding end-of-life care in heart failure. Palliat Med. 2015; 29:260–267. PMID:
25488909.
2. Yingchoncharoen T, Wu TC, Choi DJ, Ong TK, Liew HB, Cho MC. Economic burden of heart failure in Asian countries with different healthcare systems. Korean Circ J. 2021; 51:681–693. PMID:
34227265.
3. Lee HY, Oh BH. Paradigm shifts of heart failure therapy: do we need another paradigm? Int J Heart Fail. 2020; 2:145–156.
4. Choi HM, Park MS, Youn JC. Update on heart failure management and future directions. Korean J Intern Med. 2019; 34:11–43. PMID:
30612416.
5. Lee HH, Cho SM, Lee H, et al. Korea heart disease fact sheet 2020: analysis of nationwide data. Korean Circ J. 2021; 51:495–503. PMID:
34085422.
6. Park JJ, Lee CJ, Park SJ, et al. Heart failure statistics in Korea, 2020: a report from the Korean Society of Heart Failure. Int J Heart Fail. 2021; 3:224–236.
7. Alpert CM, Smith MA, Hummel SL, Hummel EK. Symptom burden in heart failure: assessment, impact on outcomes, and management. Heart Fail Rev. 2017; 22:25–39. PMID:
27592330.
8. Årestedt K, Brännström M, Evangelista LS, Strömberg A, Alvariza A. Palliative key aspects are of importance for symptom relief during the last week of life in patients with heart failure. ESC Heart Fail. 2021; 8:2202–2209. PMID:
33754461.
9. Hill L, Prager Geller T, Baruah R, et al. Integration of a palliative approach into heart failure care: a European Society of Cardiology Heart Failure Association position paper. Eur J Heart Fail. 2020; 22:2327–2339. PMID:
32892431.
10. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42:3599–3726. PMID:
34447992.
11. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2022; 79:e263–e421. PMID:
35379503.
12. Sahlollbey N, Lee CK, Shirin A, Joseph P. The impact of palliative care on clinical and patient-centred outcomes in patients with advanced heart failure: a systematic review of randomized controlled trials. Eur J Heart Fail. 2020; 22:2340–2346. PMID:
32176831.
13. Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015; 18:134–142. PMID:
25479182.
15. Crespo-Leiro MG, Metra M, Lund LH, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018; 20:1505–1535. PMID:
29806100.
16. Severino P, Mather PJ, Pucci M, et al. Advanced heart failure and end-stage heart failure: does a difference exist. Diagnostics (Basel). 2019; 9:9.
17. Maciver J, Ross HJ. A palliative approach for heart failure end-of-life care. Curr Opin Cardiol. 2018; 33:202–207. PMID:
29135524.
18. Lamont EB. A demographic and prognostic approach to defining the end of life. J Palliat Med. 2005; 8(Suppl 1):S12–S21. PMID:
16499460.
19. Kelley AS, Morrison RS. Palliative care for the seriously Ill. N Engl J Med. 2015; 373:747–755. PMID:
26287850.
20. Kida K, Doi S, Suzuki N. Palliative care in patients with advanced heart failure. Heart Fail Clin. 2020; 16:243–254. PMID:
32143768.
21. Tsutsui H, Isobe M, Ito H, et al. JCS 2017/JHFS 2017 Guideline on diagnosis and treatment of acute and chronic heart failure - digest version. Circ J. 2019; 83:2084–2184. PMID:
31511439.
22. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37:2129–2200. PMID:
27206819.
23. White N, Kupeli N, Vickerstaff V, Stone P. How accurate is the ‘Surprise Question’ at identifying patients at the end of life? A systematic review and meta-analysis. BMC Med. 2017; 15:139. PMID:
28764757.
24. White N, Reid F, Harris A, Harries P, Stone P. A systematic review of predictions of survival in palliative care: how accurate are clinicians and who are the experts? PLoS One. 2016; 11:e0161407. PMID:
27560380.
25. Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013; 34:1404–1413. PMID:
23095984.
26. Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006; 113:1424–1433. PMID:
16534009.
27. Barlera S, Tavazzi L, Franzosi MG, et al. Predictors of mortality in 6975 patients with chronic heart failure in the Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico-Heart Failure trial: proposal for a nomogram. Circ Heart Fail. 2013; 6:31–39. PMID:
23152490.
28. Pirovano M, Maltoni M, Nanni O, et al. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. J Pain Symptom Manage. 1999; 17:231–239. PMID:
10203875.
29. Morita T, Tsunoda J, Inoue S, Chihara S. The Palliative Prognostic Index: a scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer. 1999; 7:128–133. PMID:
10335930.
30. Gómez-Batiste X, Martínez-Muñoz M, Blay C, et al. Utility of the NECPAL CCOMS-ICO
© tool and the Surprise Question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: a cohort study. Palliat Med. 2017; 31:754–763. PMID:
27815556.
31. Yen YF, Lee YL, Hu HY, et al. Early palliative care: the surprise question and the palliative care screening tool-better together. BMJ Support Palliat Care. 2022; 12:211–217.
32. Libby P, Bonow R, Mann D, et al. Braunwald’s heart disease. 12th ed. Amsterdam: Elsevier;2021.
33. Lovell N, Jones C, Baynes D, Dinning S, Vinen K, Murtagh FE. Understanding patterns and factors associated with place of death in patients with end-stage kidney disease: a retrospective cohort study. Palliat Med. 2017; 31:283–288. PMID:
27495813.
34. Higginson IJ, Daveson BA, Morrison RS, et al. BuildCARE. Social and clinical determinants of preferences and their achievement at the end of life: prospective cohort study of older adults receiving palliative care in three countries. BMC Geriatr. 2017; 17:271. PMID:
29169346.
35. Tang ST, Mccorkle R. Determinants of congruence between the preferred and actual place of death for terminally ill cancer patients. J Palliat Care. 2003; 19:230–237. PMID:
14959592.
36. Nilsson J, Holgersson G, Ullenhag G, et al. Socioeconomy as a prognostic factor for location of death in Swedish palliative cancer patients. BMC Palliat Care. 2021; 20:43. PMID:
33715623.
37. Fried TR, van Doorn C, O’Leary JR, Tinetti ME, Drickamer MA. Older persons’ preferences for site of terminal care. Ann Intern Med. 1999; 131:109–112. PMID:
10419426.
38. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015; 175:827–834. PMID:
25798731.
39. ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat Rev Cardiol. 2015; 12:184–192. PMID:
25560378.
40. Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med. 2017; 377:1964–1975. PMID:
29141174.
41. O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1999; 138:78–86. PMID:
10385768.
42. Tacon CL, McCaffrey J, Delaney A. Dobutamine for patients with severe heart failure: a systematic review and meta-analysis of randomised controlled trials. Intensive Care Med. 2012; 38:359–367. PMID:
22160239.
43. Hauptman PJ, Mikolajczak P, George A, et al. Chronic inotropic therapy in end-stage heart failure. Am Heart J. 2006; 152:1096.e1–1096.e8.
44. Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009; 2:320–324. PMID:
19808355.
45. Hashim T, Sanam K, Revilla-Martinez M, et al. Clinical characteristics and outcomes of intravenous inotropic therapy in advanced heart failure. Circ Heart Fail. 2015; 8:880–886. PMID:
26179184.
46. LeMond L, Goodlin SJ. Management of heart failure in patients nearing the end of life-there is so much more to do. Card Fail Rev. 2015; 1:31–34. PMID:
28785428.
47. Ryder M, Beattie JM, O’Hanlon R, McDonald K. Multidsciplinary heart failure management and end of life care. Curr Opin Support Palliat Care. 2011; 5:317–321. PMID:
22037275.
48. Levenson JW, McCarthy EP, Lynn J, Davis RB, Phillips RS. The last six months of life for patients with congestive heart failure. J Am Geriatr Soc. 2000; 48(S1):S101–S109. PMID:
10809463.
49. Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2005; 4:198–206. PMID:
15916924.
50. Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008; 35:594–603. PMID:
18215495.
51. Ekman I, Cleland JG, Swedberg K, Charlesworth A, Metra M, Poole-Wilson PA. Symptoms in patients with heart failure are prognostic predictors: insights from COMET. J Card Fail. 2005; 11:288–292. PMID:
15880338.
52. Patel H, Shafazand M, Schaufelberger M, Ekman I. Reasons for seeking acute care in chronic heart failure. Eur J Heart Fail. 2007; 9:702–708. PMID:
17188930.
53. Tataryn D, Chochinov HM. Predicting the trajectory of will to live in terminally ill patients. Psychosomatics. 2002; 43:370–377. PMID:
12297605.
54. Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009; 54:386–396. PMID:
19628112.
55. Witte KK, Clark AL. Why does chronic heart failure cause breathlessness and fatigue? Prog Cardiovasc Dis. 2007; 49:366–384. PMID:
17329182.
56. Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001; 104:2324–2330. PMID:
11696473.
57. Booth S, Wade R, Johnson M, et al. The use of oxygen in the palliation of breathlessness. A report of the expert working group of the Scientific Committee of the Association of Palliative Medicine. Respir Med. 2004; 98:66–77. PMID:
14959816.
58. Moore DP, Weston AR, Hughes JM, Oakley CM, Cleland JG. Effects of increased inspired oxygen concentrations on exercise performance in chronic heart failure. Lancet. 1992; 339:850–853. PMID:
1347866.
59. Restrick LJ, Davies SW, Noone L, Wedzicha JA. Ambulatory oxygen in chronic heart failure. Lancet. 1992; 340:1192–1193. PMID:
1359262.
60. Chua TP, Ponikowski PP, Harrington D, Chambers J, Coats AJ. Contribution of peripheral chemoreceptors to ventilation and the effects of their suppression on exercise tolerance in chronic heart failure. Heart. 1996; 76:483–489. PMID:
9014795.
61. Russell SD, Koshkarian GM, Medinger AE, Carson PE, Higginbotham MB. Lack of effect of increased inspired oxygen concentrations on maximal exercise capacity or ventilation in stable heart failure. Am J Cardiol. 1999; 84:1412–1416. PMID:
10606114.
62. Chrysohoou C, Angelis A, Tsitsinakis G, et al. Cardiovascular effects of high-intensity interval aerobic training combined with strength exercise in patients with chronic heart failure. A randomized phase III clinical trial. Int J Cardiol. 2015; 179:269–274. PMID:
25464463.
63. Beniaminovitz A, Lang CC, LaManca J, Mancini DM. Selective low-level leg muscle training alleviates dyspnea in patients with heart failure. J Am Coll Cardiol. 2002; 40:1602–1608. PMID:
12427412.
64. Chun KH, Kang SM. Cardiac rehabilitation in heart failure. Int J Heart Fail. 2021; 3:1–14.
65. Sillen MJ, Speksnijder CM, Eterman RA, et al. Effects of neuromuscular electrical stimulation of muscles of ambulation in patients with chronic heart failure or COPD: a systematic review of the English-language literature. Chest. 2009; 136:44–61. PMID:
19363213.
66. Ennis S, McGregor G, Hamborg T, et al. Randomised feasibility trial into the effects of low-frequency electrical muscle stimulation in advanced heart failure patients. BMJ Open. 2017; 7:e016148.
67. Bausewein C, Booth S, Gysels M, Higginson I. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev. 2008; (2):CD005623. PMID:
18425927.
68. Williams SG, Wright DJ, Marshall P, et al. Safety and potential benefits of low dose diamorphine during exercise in patients with chronic heart failure. Heart. 2003; 89:1085–1086. PMID:
12923038.
69. Johnson MJ, McDonagh TA, Harkness A, McKay SE, Dargie HJ. Morphine for the relief of breathlessness in patients with chronic heart failure--a pilot study. Eur J Heart Fail. 2002; 4:753–756. PMID:
12453546.
70. Chua TP, Harrington D, Ponikowski P, Webb-Peploe K, Poole-Wilson PA, Coats AJ. Effects of dihydrocodeine on chemosensitivity and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 1997; 29:147–152. PMID:
8996307.
71. Godfrey C, Harrison MB, Medves J, Tranmer JE. The symptom of pain with heart failure: a systematic review. J Card Fail. 2006; 12:307–313. PMID:
16679265.
72. Angermann CE, Gelbrich G, Störk S, et al. Somatic correlates of comorbid major depression in patients with systolic heart failure. Int J Cardiol. 2011; 147:66–73. PMID:
19716613.
73. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006; 48:1527–1537. PMID:
17045884.
74. Morgan K, Villiers-Tuthill A, Barker M, McGee H. The contribution of illness perception to psychological distress in heart failure patients. BMC Psychol. 2014; 2:50. PMID:
25520809.
75. Blumenthal JA, Babyak MA, O’Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA. 2012; 308:465–474. PMID:
22851113.
76. Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med. 2007; 120:799–806. PMID:
17765050.
77. Tu RH, Zeng ZY, Zhong GQ, et al. Effects of exercise training on depression in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Eur J Heart Fail. 2014; 16:749–757. PMID:
24797230.
78. Gottlieb SS, Kop WJ, Thomas SA, et al. A double-blind placebo-controlled pilot study of controlled-release paroxetine on depression and quality of life in chronic heart failure. Am Heart J. 2007; 153:868–873. PMID:
17452166.
79. O’Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010; 56:692–699. PMID:
20723799.
80. Angermann CE, Gelbrich G, Störk S, et al. Effect of escitalopram on All-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016; 315:2683–2693. PMID:
27367876.
81. Schaefer KM, Shober Potylycki MJ. Fatigue associated with congestive heart failure: use of Levine’s Conservation Model. J Adv Nurs. 1993; 18:260–268. PMID:
8436716.
82. Blinderman CD, Billings JA. Comfort care for patients dying in the hospital. N Engl J Med. 2015; 373:2549–2561. PMID:
26699170.
83. Glare P, Miller J, Nikolova T, Tickoo R. Treating nausea and vomiting in palliative care: a review. Clin Interv Aging. 2011; 6:243–259. PMID:
21966219.
84. Bekfani T, Pellicori P, Morris DA, et al. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol. 2016; 222:41–46. PMID:
27454614.
85. Vitale C, Jankowska E, Hill L, et al. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail. 2019; 21:1299–1305. PMID:
31646718.
86. Saitoh M, Ishida J, Doehner W, et al. Sarcopenia, cachexia, and muscle performance in heart failure: review update 2016. Int J Cardiol. 2017; 238:5–11. PMID:
28427849.
87. Veninšek G, Gabrovec B. Management of frailty at individual level - clinical management: systematic literature review. Zdr Varst. 2018; 57:106–115. PMID:
29651322.
88. Sumukadas D, Struthers AD, McMurdo ME. Sarcopenia--a potential target for Angiotensin-converting enzyme inhibition? Gerontology. 2006; 52:237–242. PMID:
16849867.
89. Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995; 50(Special):5–8. PMID:
7493218.
90. Quill TE, Lo B, Brock DW, Meisel A. Last-resort options for palliative sedation. Ann Intern Med. 2009; 151:421–424. PMID:
19755367.
91. Cherny NI, Radbruch L. Board of the European Association for Palliative Care. European Association for Palliative Care (EAPC) recommended framework for the use of sedation in palliative care. Palliat Med. 2009; 23:581–593. PMID:
19858355.
92. Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol. 2017; 70:331–341. PMID:
28705314.
93. Puchalski C, Ferrell B, Virani R, et al. Improving the quality of spiritual care as a dimension of palliative care: the report of the Consensus Conference. J Palliat Med. 2009; 12:885–904. PMID:
19807235.
94. El Nawawi NM, Balboni MJ, Balboni TA. Palliative care and spiritual care: the crucial role of spiritual care in the care of patients with advanced illness. Curr Opin Support Palliat Care. 2012; 6:269–274. PMID:
22469668.
95. Puchalski CM. The FICA spiritual history tool #274. J Palliat Med. 2014; 17:105–106. PMID:
24351125.
96. Garlo K, O’Leary JR, Van Ness PH, Fried TR. Burden in caregivers of older adults with advanced illness. J Am Geriatr Soc. 2010; 58:2315–2322. PMID:
21087225.
97. McIlvennan CK, Jones J, Allen LA, Swetz KM, Nowels C, Matlock DD. Bereaved caregiver perspectives on the end-of-life experience of patients with a left ventricular assist device. JAMA Intern Med. 2016; 176:534–539. PMID:
26998594.
98. Waller A, Turon H, Mansfield E, Clark K, Hobden B, Sanson-Fisher R. Assisting the bereaved: a systematic review of the evidence for grief counselling. Palliat Med. 2016; 30:132–148. PMID:
26415735.
99. Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015; 372:153–160. PMID:
25564898.
100. Park KH, Lee CH, Jung BC, et al. Effectiveness of implantable cardioverter-defibrillator therapy for heart failure patients according to ischemic or non-ischemic etiology in Korea. Korean Circ J. 2017; 47:72–81. PMID:
28154594.
101. Pettit SJ, Browne S, Hogg KJ, et al. ICDs in end-stage heart failure. BMJ Support Palliat Care. 2012; 2:94–97.
102. Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med. 2004; 141:835–838. PMID:
15583224.
103. Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017; 36:1080–1086. PMID:
28942782.
104. Nakatani T, Sase K, Oshiyama H, et al. Japanese registry for Mechanically assisted circulatory support: first report. J Heart Lung Transplant. 2017; 36:1087–1096. PMID:
28942783.
105. Shapiro PA, Levin HR, Oz MC. Left ventricular assist devices. Psychosocial burden and implications for heart transplant programs. Gen Hosp Psychiatry. 1996; 18(Suppl):30S–35S. PMID:
8937921.
106. Dunlay SM, Strand JJ, Wordingham SE, Stulak JM, Luckhardt AJ, Swetz KM. Dying with a left ventricular assist device as destination therapy. Circ Heart Fail. 2016; 9:e003096. PMID:
27758809.
107. Okumura T, Sawamura A, Murohara T. Palliative and end-of-life care for heart failure patients in an aging society. Korean J Intern Med. 2018; 33:1039–1049. PMID:
29779361.
108. Pope TM, Hexum M. Legal briefing: POLST: physician orders for life-sustaining treatment. J Clin Ethics. 2012; 23:353–376. PMID:
23469698.
109. Guidelines for Emergency Physicians on the Interpretation of Physician Orders for Life-Sustaining Therapy (POLST). Guidelines for Emergency Physicians on the Interpretation of Physician Orders for Life-Sustaining Therapy (POLST). Ann Emerg Med. 2017; 70:122–125. PMID:
28645409.
110. Burns JP, Truog RD. The DNR order after 40 years. N Engl J Med. 2016; 375:504–506. PMID:
27509098.
111. Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010; 362:1173–1180. PMID:
20357280.
112. Kim HL, Jung MH, Choi JH, et al. Factors associated with low awareness of heart failure in the general population of Korea. Korean Circ J. 2020; 50:586–595. PMID:
32212427.
113. Brunner-La Rocca HP, Rickenbacher P, Muzzarelli S, et al. End-of-life preferences of elderly patients with chronic heart failure. Eur Heart J. 2012; 33:752–759. PMID:
22067089.
114. Schichtel M, Wee B, Perera R, Onakpoya I, Albury C, Barber S. Clinician-targeted interventions to improve advance care planning in heart failure: a systematic review and meta-analysis. Heart. 2019; 105:1316–1324. PMID:
31118199.
115. Kavalieratos D, Gelfman LP, Tycon LE, et al. Palliative care in heart failure: rationale, evidence, and future priorities. J Am Coll Cardiol. 2017; 70:1919–1930. PMID:
28982506.
116. Baek J, Lee H, Lee HH, Heo JE, Cho SM, Kim HC. Thirty-six year trends in mortality from diseases of circulatory system in Korea. Korean Circ J. 2021; 51:320–332. PMID:
33821581.
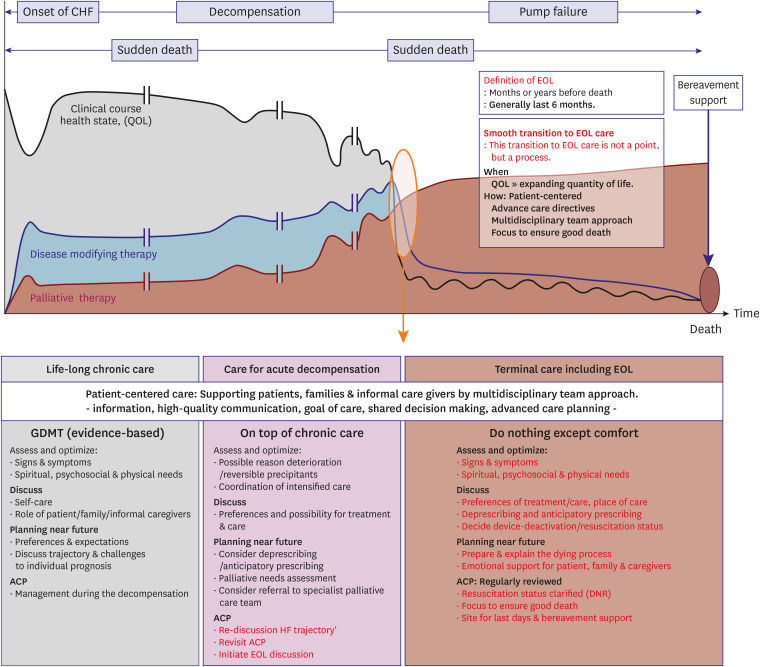
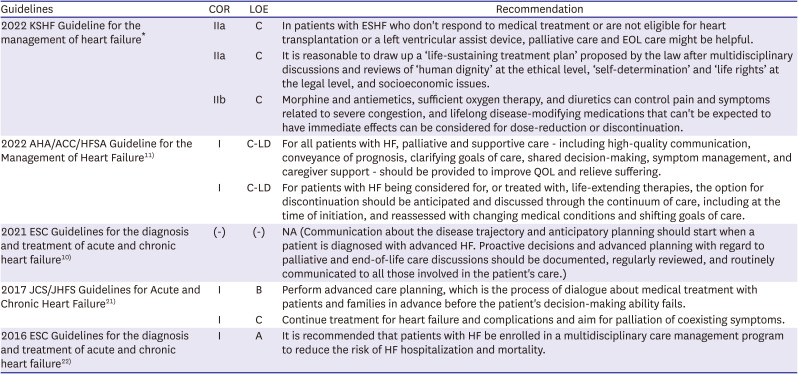
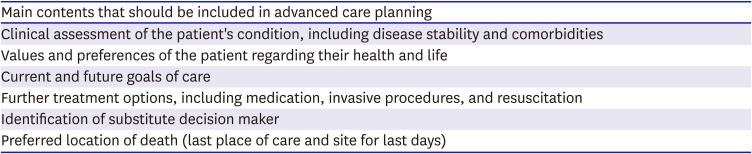

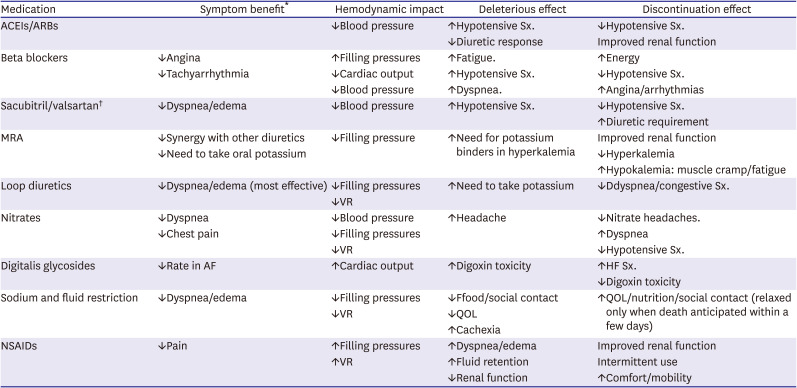





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download