Introduction
Materials and methods
1. Participants
2. Study participants
3. Statistical analysis
Results
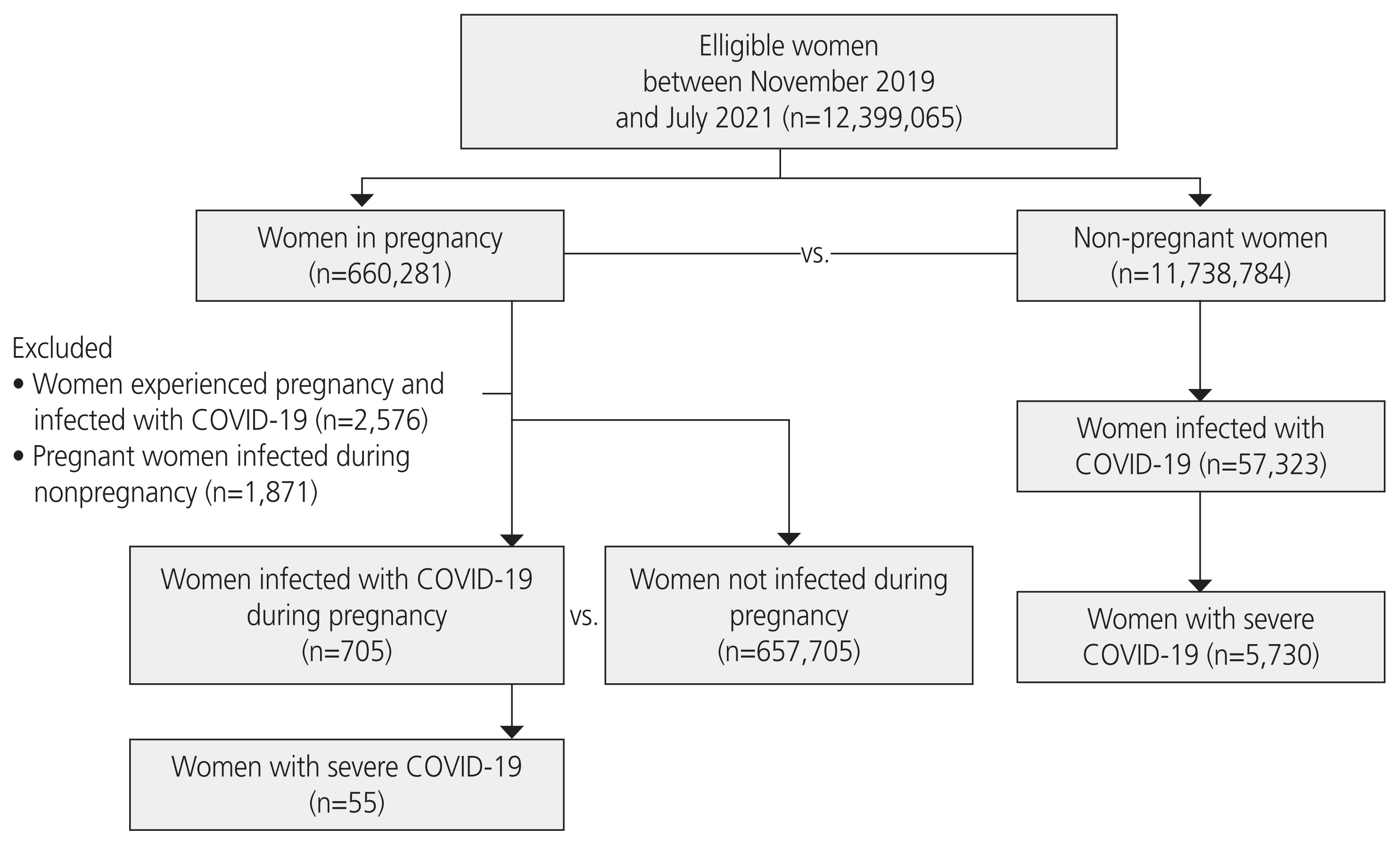 | Fig. 1Flowchart of inclusion of women based on pregnancy and COVID-19 status. COVID-19, coronavirus disease 2019. |
Table 1
| Total women (n=12,397,194a)) | |
|---|---|
| Pregnancy, yes | |
| COVID-19, yes | 705 (0.11%) |
| COVID-19, no | 657,705 (99.89%) |
| Total | 658,410 (5.31% of total women) |
| Pregnancy, no | |
| COVID-19, yes | 57,323 (0.49%) |
| COVID-19, no | 11,681,461 (99.51%) |
| Total | 11,738,784 (94.68% of total women) |
Table 2-1
| Variable | Diagnosis | Pregnant women without COVID-19 (n=657,705) | Pregnant women with COVID-19 (n=705) | P-value |
|---|---|---|---|---|
| O00 | Ectopic pregnancy | 4,350 (0.66) | 5 (0.71) | >0.9999 |
| O01 | Hydatidiform mole | 879 (0.13) | 0 (0.00) | >0.9999 |
| O02 | Other abnormal products of conception | 60,754 (9.24) | 94 (13.33) | 0.0002a) |
| O03 | Spontaneous abortion | 10,838 (1.65) | 17 (2.41) | 0.1490 |
| O04 | Complications following (induced) termination of pregnancy | 754 (0.11) | 1 (0.14) | 0.5548 |
| O05 | Other abortion | 1,093 (0.17) | 0 (0.00) | 0.6365 |
| O06 | Unspecified abortion | 1,813 (0.28) | 3 (0.43) | 0.4508 |
| O07 | Failed attempted termination of pregnancy | 29 (0.00) | 0 (0.00) | >0.9999 |
| O08 | Complications following ectopic and molar pregnancy | 4,902 (0.75) | 3 (0.43) | 0.5052 |
| O10 | Pre-existing hypertension complicating pregnancy, childbirth and the puerperium | 2,946 (0.45) | 3 (0.43) | >0.9999 |
| O11 | Pre-existing hypertensive disorder with superimposed proteinuria | 770 (0.12) | 1 (0.14) | 0.5624 |
| O12 | Gestational (pregnancy-induced) edema and proteinuria without hypertension | 143,983 (21.89) | 91 (12.91) | <0.0001a) |
| O13 | Gestational (pregnancy-induced) hypertension without significant proteinuria | 17,210 (2.62) | 13 (1.84) | 0.2433 |
| O14 | Gestational (pregnancy-induced) hypertension with significant proteinuria | 9,064 (1.38) | 8 (1.13) | 0.6948 |
| O15 | Eclampsia | 233 (0.04) | 0 (0.00) | >0.9999 |
| O16 | Unspecified maternal hypertension | 6,881 (1.05) | 9 (1.28) | 0.6777 |
| O20 | Hemorrhage in early pregnancy | 184,568 (28.06) | 206 (29.22) | 0.5211 |
| O21 | Excessive vomiting in pregnancy | 128,205 (19.49) | 144 (20.43) | 0.5638 |
| O22 | Venous complications in pregnancy | 138,062 (20.99) | 100 (14.18) | <0.0001a) |
| O23 | Infection of genitourinary tract in pregnancy | 153,156 (23.29) | 114 (16.17) | <0.0001a) |
| O24 | Diabetes mellitus in pregnancy | 231,823 (35.25) | 184 (26.1) | <0.0001a) |
| O25 | Malnutrition in pregnancy | 442 (0.07) | 1 (0.14) | 0.3780 |
| O26 | Maternal care for other conditions predominantly related to pregnancy | 33,796 (5.14) | 27 (3.83) | 0.1368 |
| O28 | Abnormal findings on antenatal screening of mother | 17,564 (2.67) | 12 (1.70) | 0.1396 |
| O29 | Complications of anesthesia during pregnancy | 92 (0.01) | 0 (0.00) | >0.9999 |
| O30 | Multiple gestation | 23,485 (3.57) | 31 (4.4) | 0.2800 |
| O31 | Complications specific to multiple gestation | 1,587 (0.24) | 1 (0.14) | >0.9999 |
| O32 | Maternal care for known or suspected malpresentation of fetus | 39,625 (6.02) | 29 (4.11) | 0.0401a) |
| O33 | Maternal care for known or suspected disproportion | 71,341 (10.85) | 28 (3.97) | <0.0001a) |
| O34 | Maternal care for known or suspected abnormality of pelvic organs | 126,536 (19.24) | 119 (16.88) | 0.1234 |
| O35 | Maternal care for known or suspected fetal abnormality and damage | 17,257 (2.62) | 13 (1.84) | 0.2392 |
| O36 | Maternal care for other known or suspected fetal problems | 41,246 (6.27) | 30 (4.26) | 0.0332a) |
| O30 | Multiple gestation | 23,485 (3.57) | 31 (4.4) | 0.2800 |
| O31 | Complications specific to multiple gestation | 1,587 (0.24) | 1 (0.14) | >0.9999 |
| O32 | Maternal care for known or suspected malpresentation of fetus | 39,625 (6.02) | 29 (4.11) | 0.0401a) |
| O33 | Maternal care for known or suspected disproportion | 71,341 (10.85) | 28 (3.97) | <0.0001a) |
| O34 | Maternal care for known or suspected abnormality of pelvic organs | 126,536 (19.24) | 119 (16.88) | 0.1234 |
| O35 | Maternal care for known or suspected fetal abnormality and damage | 17,257 (2.62) | 13 (1.84) | 0.2392 |
| O36 | Maternal care for other known or suspected fetal problems | 41,246 (6.27) | 30 (4.26) | 0.0332a) |
| O40 | Polyhydramnios | 5,338 (0.81) | 4 (0.57) | 0.6717 |
| O41 | Other disorders of amniotic fluid and membranes | 28,652 (4.36) | 23 (3.26) | 0.1835 |
| O42 | Premature rupture of membranes | 101,828 (15.48) | 67 (9.5) | 0.0000a) |
| O43 | Placental disorders | 3,597 (0.55) | 1 (0.14) | 0.1974 |
| O44 | Placenta previa | 19,580 (2.98) | 20 (2.84) | 0.9140 |
| O45 | Premature separation of placenta (abruptio placentae) | 2,393 (0.36) | 3 (0.43) | 0.7466 |
| O46 | Antepartum hemorrhage, NEC | 18,190 (2.77) | 12 (1.7) | 0.1082 |
| O47 | False labor | 73,269 (11.14) | 45 (6.38) | 0.0001a) |
| O48 | Prolonged pregnancy | 3,062 (0.47) | 1 (0.14) | 0.3936 |
| O60 | Preterm delivery | 140,953 (21.43) | 124 (17.59) | 0.0147a) |
| O61 | Failed induction of labor | 18,538 (2.82) | 13 (1.84) | 0.1473 |
| O62 | Abnormalities of forces of labor | 11,982 (1.82) | 8 (1.13) | 0.2215 |
| O63 | Long labor | 17,483 (2.66) | 6 (0.85) | 0.0042a) |
| O64 | Obstructed labor due to malposition and malpresentation of fetus | 3,686 (0.56) | 1 (0.14) | 0.1993 |
| O65 | Obstructed labor due to maternal pelvic abnormality | 36,981 (5.62) | 15 (2.13) | 0.0001a) |
| O66 | Other obstructed labor | 12,729 (1.94) | 4 (0.57) | 0.0038a) |
| O67 | Labor and delivery complicated by intrapartum hemorrhage, NEC | 4,923 (0.75) | 0 (0.00) | 0.0128a) |
| O68 | Labor and delivery complicated by fetal stress (distress) | 23,397 (3.56) | 16 (2.27) | 0.0812 |
| O69 | Labor and delivery complicated by umbilical cord complications | 3,336 (0.51) | 4 (0.57) | 0.7862 |
| O70 | Perineal laceration during delivery | 42,428 (6.45) | 15 (2.13) | <0.0001a) |
| O71 | Other obstetric trauma | 11,043 (1.68) | 3 (0.43) | 0.0048a) |
| O72 | Postpartum hemorrhage | 62,532 (9.51) | 23 (3.26) | <0.0001a) |
| O73 | Retained placenta and membranes, without hemorrhage | 2,076 (0.32) | 1 (0.14) | 0.7320 |
| O74 | Complications of anesthesia during labor and delivery | 361 (0.05) | 0 (0.00) | >0.9999 |
| O75 | Other complications of labor and delivery, NEC | 3,852 (0.59) | 0 (0.00) | 0.0408a) |
| O80 | Single spontaneous delivery | 146,224 (22.23) | 72 (10.21) | <0.0001a) |
| O81 | Single delivery by forceps and vacuum extractor | 21,636 (3.29) | 8 (1.13) | 0.0019a) |
| O82 | Single delivery by cesarean section | 222,780 (33.87) | 157 (22.27) | <0.0001a) |
| O83 | Other assisted single delivery | 32,734 (4.98) | 18 (2.55) | 0.0041a) |
| O84 | Multiple delivery | 7,494 (1.14) | 4 (0.57) | 0.2092 |
| O85 | Puerperal sepsis | 595 (0.09) | 1 (0.14) | 0.4721 |
| O86 | Other puerperal infections | 65,996 (10.03) | 19 (2.7) | 0.0000a) |
| O87 | Venous complications during puerperium | 61,483 (9.35) | 36 (5.11) | 0.0001a) |
| O88 | Obstetric embolism | 132 (0.02) | 0 (0.00) | >0.9999 |
| O89 | Complications of anesthesia during the puerperium | 559 (0.08) | 0 (0.00) | >0.9999 |
| O90 | Complications of the puerperium, NEC | 29,280 (4.45) | 10 (1.42) | 0.0001a) |
| O91 | Infections of the breast associated with childbirth | 17,045 (2.59) | 5 (0.71) | 0.0025a) |
| O92 | Other disorders of the breast and lactation associated with childbirth | 8,306 (1.26) | 0 (0.00) | 0.0003a) |
| O94 | Sequelae of complication of pregnancy, childbirth and the puerperium | 237,195 (36.06) | 218 (30.92) | 0.0051a) |
Table 2-2
| Variable | Pregnant women without COVID-19 (n=657,705) | Pregnant women with COVID-19 (n=705) | P-value |
|---|---|---|---|
| Preterm birth | 98,227 (14.93) | 76 (10.78) | 0.0024a) |
| Cesarean section | 232,849 (35.40) | 133 (18.87) | <0.0001a) |
Table 2-3
| Total women (n=12,397,194a)) | |
|---|---|
| Pregnancy, yes | |
| Severe COVID-19, yes | 55 (7.80) |
| Severe COVID-19, no | 650 (92.20) |
| Pregnancy, no | |
| Severe COVID-19, yes | 5,730 (10.00) |
| Severe COVID-19, no | 51,593 (90.00) |
Table 3-1
| Variable | Diagnosis | Pregnant women without COVID-19 (n=336,114) | Pregnant women with COVID-19 (n=139) | P-value |
|---|---|---|---|---|
| P00 | Fetus and newborn affected by maternal conditions that may be unrelated to present pregnancy | 9,852 (2.93) | 22 (15.83) | <0.0001a) |
| P01 | Fetus and newborn affected by maternal complications of pregnancy | 9,859 (2.93) | 4 (2.88) | 0.9999 |
| P02 | Fetus and newborn affected by maternal complications of placenta, cord and membranes | 2,549 (0.76) | 0 (0.00) | 0.6312 |
| P03 | Fetus and newborn affected by other complications of labor and delivery | 7,296 (2.17) | 10 (7.19) | <0.0001a) |
| P04 | Fetus and newborn affected by noxious influences transmitted via the placenta or breast milk | 1,530 (0.46) | 0 (0.00) | >0.9999 |
| P05 | Slow fetal growth and fetal malnutrition | 3,392 (1.01) | 3 (2.16) | 0.1666 |
| P07 | Disorders related to short gestation and low birth weight, NEC | 21,796 (6.48) | 14 (10.07) | 0.1224 |
| P08 | Disorders related to long gestation and high birth weight | 4,429 (1.32) | 1 (0.72) | >0.9999 |
| P10 | Intracranial laceration and hemorrhage due to birth injury | 101 (0.03) | 0 (0.00) | >0.9999 |
| P11 | Other birth injuries to central nervous system | 30 (0.01) | 0 (0.00) | >0.9999 |
| P12 | Birth injury to scalp | 3,114 (0.93) | 4 (2.88) | 0.0412a) |
| P13 | Birth injury to skeleton | 3,405 (1.01) | 1 (0.72) | >0.9999 |
| P14 | Birth injury to peripheral nervous system | 50 (0.01) | 0 (0.00) | >0.9999 |
| P15 | Other birth injuries | 90 (0.03) | 0 (0.00) | >0.9999 |
| P20 | Intrauterine hypoxia | 980 (0.29) | 1 (0.72) | 0.3338 |
| P21 | Birth asphyxia | 653 (0.19) | 0 (0.00) | >0.9999 |
| P22 | Respiratory distress of newborn | 27,571 (8.2) | 17 (12.23) | 0.1152 |
| P23 | Congenital pneumonia | 1,193 (0.35) | 0 (0.00) | >0.9999 |
| P24 | Neonatal aspiration syndromes | 12,067 (3.59) | 5 (3.6) | >0.9999 |
| P25 | Interstitial emphysema and related conditions originating in the perinatal period | 715 (0.21) | 0 (0.00) | >0.9999 |
| P26 | Pulmonary hemorrhage originating in the perinatal period | 88 (0.03) | 0 (0.00) | >0.9999 |
| P27 | Chronic respiratory disease originating in the perinatal period | 654 (0.19) | 0 (0.00) | >0.9999 |
| P28 | Other respiratory conditions originating in the perinatal period | 8,980 (2.67) | 4 (2.88) | 0.7899 |
| P29 | Cardiovascular disorders originating in the perinatal period | 1,992 (0.59) | 0 (0.00) | >0.9999 |
| P35 | Congenital viral diseases | 710 (0.21) | 0 (0.00) | >0.9999 |
| P36 | Bacterial sepsis of newborn | 4,657 (1.39) | 2 (1.44) | 0.7192 |
| P37 | Other congenital infectious and parasitic diseases | 235 (0.07) | 0 (0.00) | >0.9999 |
| P38 | Omphalitis of newborn with or without mild hemorrhage | 29,331 (8.73) | 16 (11.51) | 0.3113 |
| P39 | Other infections specific to the perinatal period | 25,291 (7.52) | 15 (10.79) | 0.1940 |
| P50 | Fetal blood loss | 67 (0.02) | 0 (0.00) | >0.9999 |
| P51 | Umbilical hemorrhage of newborn | 1,475 (0.44) | 1 (0.72) | 0.4575 |
| P52 | Intracranial nontraumatic hemorrhage of fetus and newborn | 1,559 (0.46) | 1 (0.72) | 0.4761 |
| P53 | Hemorrhagic disease of fetus and newborn | 1,016 (0.30) | 0 (0.00) | >0.9999 |
| P54 | Other neonatal hemorrhages | 1,025 (0.30) | 2 (1.44) | 0.0680 |
| P55 | Hemolytic disease of fetus and newborn | 1,132 (0.34) | 0 (0.00) | >0.9999 |
| P56 | Hydrops fetalis due to hemolytic disease | 5 (0.00) | 0 (0.00) | >0.9999 |
| P57 | Kernicterus | 58 (0.02) | 0 (0.00) | >0.9999 |
| P58 | Neonatal jaundice due to other excessive hemolysis | 1,128 (0.34) | 2 (1.44) | 0.0801 |
| P59 | Neonatal jaundice from other and unspecified causes | 133,504 (39.72) | 57 (41.01) | 0.8232 |
| P60 | Disseminated intravascular coagulation of fetus and newborn | 308 (0.09) | 0 (0.00) | >0.9999 |
| P61 | Other perinatal hematological disorders | 2,425 (0.72) | 1 (0.72) | >0.9999 |
| P70 | Transitory disorders of carbohydrate metabolism specific to fetus and newborn | 25,904 (7.71) | 9 (6.47) | 0.6999 |
| P71 | Transitory neonatal disorders of calcium and magnesium metabolism | 3,990 (1.19) | 1 (0.72) | >0.9999 |
| P72 | Other transitory neonatal endocrine disorders | 6,748 (2.01) | 6 (4.32) | 0.1015 |
| P74 | Other transitory neonatal electrolyte and metabolic disturbances | 3,552 (1.06) | 3 (2.16) | 0.1828 |
| P75 | Meconium ileus | 3 (0.00) | 0 (0.00) | >0.9999 |
| P76 | Other intestinal obstruction of newborn | 256 (0.08) | 0 (0.00) | >0.9999 |
| P77 | Necrotizing enterocolitis of fetus and newborn | 437 (0.13) | 0 (0.00) | >0.9999 |
| P78 | Other perinatal digestive system disorders | 3,891 (1.16) | 2 (1.44) | 0.6773 |
| P80 | Hypothermia of newborn | 154 (0.05) | 0 (0.00) | >0.9999 |
| P81 | Other disturbances of temperature regulation of newborn | 1,661 (0.49) | 2 (1.44) | 0.1512 |
| P83 | Other conditions of integument specific to fetus and newborn | 12,061 (3.59) | 3 (2.16) | 0.4948 |
| P90 | Convulsions of newborn | 659 (0.20) | 0 (0.00) | >0.9999 |
| P91 | Other disturbances of cerebral status of newborn | 1,130 (0.34) | 0 (0.00) | >0.9999 |
| P92 | Feeding problems of newborn | 13,302 (3.96) | 4 (2.88) | 0.6651 |
| P93 | Reactions and intoxications due to drugs administered to fetus and newborn | 1 (0.00) | 0 (0.00) | >0.9999 |
| P94 | Disorders of muscle tone of newborn | 117 (0.03) | 0 (0.00) | >0.9999 |
| P95 | Fetal death of unspecified cause | 0 (0.00) | 0 (0.00) | >0.9999 |
| P96 | Other conditions originating in the perinatal period | 5,060 (1.51) | 2 (1.44) | >0.9999 |
Table 3-2
| Variable | Pregnant women without COVID-19 (n=336,114) | Pregnant women with COVID-19 (n=139) | P-value |
|---|---|---|---|
| Low birth weight | |||
| 1,000–1,249 g | 114 (0.03) | 0 (0.00) | >0.9999 |
| 1,250–1,499 g | 403 (0.12) | 0 (0.00) | >0.9999 |
| 1,500–1,749 g | 993 (0.30) | 0 (0.00) | >0.9999 |
| 1,750–1,999 g | 2,173 (0.65) | 1 (0.72) | 0.5942 |
| 2,000–2,499 g | 8,420 (2.51) | 6 (4.32) | 0.2737 |
| Etc. | 112 (0.03) | 0 (0.00) | >0.9999 |
| Unknown weight | 1,513 (0.45) | 0 (0.00) | >0.9999 |
| Total | 13,728 (4.08) | 7 (5.04) | 0.7245 |
| Macrosomia | 4,429 (1.32) | 1 (0.72) | >0.9999 |
| NICU | 25,971 (7.73) | 26 (18.71) | <0.0001a) |
Table 4-1
| Moderna | Janssen | AstraZeneca | Pfizer | |
|---|---|---|---|---|
| Vaccination during pregnancy | ||||
| No | 34,504 | 7,418 | 14,634 | 195,131 |
| Yes | 1,734 | 439 | 1,135 | 10,132 |
Table 4-2
Table 5
O00–O08, pregnancy with abortive outcome; O10–O16, edema, proteinuria, and hypertensive disorders in pregnancy, childbirth, and puerperium; O20–O29, other maternal disorders predominantly related to pregnancy; O30–O48, maternal care related to the fetus and amniotic cavity and possible delivery problems; O60–O75, complications of labor and delivery; O80–O84, encounter for delivery; O85–O92, complications predominantly related to puerperium; O94–O99, other obstetric conditions, not elsewhere classified.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download