Abstract
Objectives
Symptomatic treatment is an essential component in the overall treatment of multiple sclerosis (MS). However, knowledge in this regard is confusing and scattered. Physicians also have challenges in choosing symptomatic treatment based on the patient’s condition. To share, update, and reuse this knowledge, the aim of this study was to provide an ontology for MS symptomatic treatment.
Methods
The Symptomatic Treatment of Multiple Sclerosis Ontology (STMSO) was developed according to Ontology Development 101 and a guideline for developing good ontologies in the biomedical domain. We obtained knowledge and rules through a systematic review and entered this knowledge in the form of classes and subclasses in the ontology. We then mapped the ontology using the Basic Formal Ontology (BFO) and Ontology for General Medical Sciences (OGMS) as reference ontologies. The ontology was built using Protégé Editor in the Web Ontology Language format. Finally, an evaluation was done by experts using criterion-based approaches in terms of accuracy, clarity, consistency, and completeness.
Results
The knowledge extraction phase identified 110 articles related to the ontology in the form of 626 classes, 40 object properties, and 139 rules. Five general classes included “patient,” “symptoms,” “pharmacological treatment,” “treatment plan,” and “measurement index.” The evaluation in terms of standards for biomedical ontology showed that STMSO was accurate, clear, consistent, and complete.
Multiple sclerosis (MS) is an autoimmune inflammatory disease of the central nervous system that can cause progressive disability in young adults [1]. There are more than 2.3 million MS patients worldwide, who encounter numerous symptoms such as spasticity, pain, bladder dysfunction, fatigue, and emotional disturbances [2]. These symptoms may have a major impact on their daily function and quality of life.
There is no cure for MS, and treatments are typically focused on slowing the progression of the disease (disease-modifying treatments) and alleviating symptoms [3]. In this regard, clinical approaches for follow-up appointments should pay special attention to symptom-targeted treatments in MS patients with either stable or severe progressive courses. Symptomatic treatment accounts for a significant part of the physician’s role and is an important part of a comprehensive treatment plan that aims to improve MS patients’ quality of life [4].
Comprehensive care for chronic diseases, including MS, involves a combination of both pharmacological and non-pharmacological interventions [4,5]. Pharmacological interventions aim to reduce MS-related functional and emotional impairment [5]. Non-pharmacological interventions, such as psychological interventions, education, and rehabilitation, may be provided as an adjunct to medical treatment [4].
In routine clinical practice, physicians face challenges in choosing distinct interventions that are compatible with the patient’s conditions, such as age, comorbidities, the severity of symptoms, lifestyle, or special conditions such as pregnancy [6]. The ultimate goal in MS symptomatic treatment is to select the most suitable pharmacological and non-pharmacological treatment depending on the needs expressed by patients [7]. Making tailored treatment decisions for specific patients is challenging due to the need to check many interrelated symptoms and medications that may interact with each other [8]. Simultaneously, there is a large amount of scattered practical information in clinical bulletins and articles about pharmacological and non-pharmacological interventions that could support the physician’s decision. However, the existing information contains lengthy unstructured text data that are in natural language and cannot be interpreted by a computer, and it is challenging for physicians to retrieve and integrate this scattered information to make the right decision [9]. That consequently wastes physicians’ time and energy.
Given the challenges of using scattered information, the most effective solution is to use an ontology that could remove the barriers related to the heterogeneity of knowledge by integrating this information and transforming unstructured text data into structured data [10]. Ontologies can provide a knowledge base for clinical decision support systems (CDSS), preserve the semantic relationships between MS treatment concepts, and improve the intelligence of CDSS [11].
The reasoning mechanism of any knowledge-based system is vital. An ontology supports reasoning to recommend a suitable treatment by considering the medical status of the patient. Many clinical reasoning ontologies have been developed to assist in the treatment of some chronic diseases, such as cancer [12] and diabetes [13]. Given the importance of making correct clinical decisions for symptomatic treatment in the management of MS patients, an ontology could provide a knowledge base for semantically intelligent CDSS in this domain.
Previous studies have applied some ontologies in the field of MS. For example, Malhotra et al. [14], developed the MS ontology semantic framework, which was able to automatically extract information from both the scientific literature and Electronic Medical Records (EMRs). However, that study lacked sufficient information on the symptomatic treatment of MS patients. Another study that focused on the symptomatic treatment of certain neurological diseases [15] did not specifically address MS as a distinct neurological disorder that presents a special constellation of motor, sensory and cognitive symptoms. In a registry-based study, Jensen et al. [16] compared patient-reported outcomes (e.g., disability and quality of life) with objective outcomes derived from clinical examinations. That study was not able to provide ontological information about different treatment modalities. A comprehensive ontological study addressing different concepts of MS symptomatic treatment is lacking. Therefore, the objective of the study was to develop an ontology of symptomatic treatment in MS care as a knowledge base for developing CDSS in this domain.
This was a descriptive developmental study that addressed the development and evaluation process of the Symptomatic Treatment of Multiple Sclerosis Ontology (STMSO). Before conducting the research, ethical approval was obtained from the National Ethics Committee for Biomedical Research (No. IR.IUMS.REC.1398.1208).
Considering the multidisciplinary nature of symptomatic treatment, two groups of experts were invited to participate in this study: neurologists and clinicians. The first group included four neurologists, of whom two were faculty members with more than 10 years of experience in the treatment of MS and the other two were researchers in the field of neurology. The second group included a consultant who had a master’s degree in psychology and an experienced physiotherapist. All participants worked at the MS clinics of two university hospitals (Kashani and Al-Zahra) in Esfahan, Iran.
This study had two main phases: ontology development and ontology evaluation. The development process for creating the STMSO consisted of six main steps that were carried out according to Ontology Development 101 [17] and a guideline on developing good ontologies in the biomedical domain [18]. The method for building STMSO is illustrated in Figure 1. A detailed description of each component of the method is presented next.
This step determined the requirements that the STMSO needed to meet, which included information about the purpose, scope, and competency questions (CQs). The proposed CQs were defined through a literature review and brain-storming with domain experts. These CQs guided the next phases of ontology development. The STMSO must have the ability to answer these questions in line with the users’ needs. The answers to these questions helped the developers to identify the essential information to build this ontology.
The purpose of this step was to acquire knowledge about MS symptomatic treatment terms and rules to build a complete ontology. The knowledge was extracted by performing a systematic review and consulting with domain experts.
The literature was searched from 2010 to 2021 using combinations of the following keywords: “multiple sclerosis,” “symptomatic drug,” “symptomatic medication,” “symptomatic therapy,” “symptomatic management,” and “symptomatic treatment.” The selection criterion was papers that proposed MS treatments. We also searched MS-related websites, including the National Multiple Sclerosis Society and the National Guideline Clearinghouse, to extract structured knowledge relevant to concepts, properties, and rules. Based on the selected literature, a systematic knowledge elicitation approach was performed to extract terms for representing knowledge in the domain of MS symptomatic treatment. This process was undertaken manually by the first author in consultation with two other authors. Through careful reading of the selected literature, the key concepts and their interrelationships in the MS symptomatic domain were extracted and documented in a checklist. Table 1 shows an excerpt of this checklist.
Excerpt of the checklist
In this step, rules were also defined. Rules address a number of challenges in symptomatic treatment. First, we gathered information through informal meetings with neurologist and clinician experts, analysis of the literature, and investigations of related websites such as the Medication Guide of the US Food and Drug Administration [19] and the “DrugBank” online database. These challenges were then formulated in the form of “if…then” clauses. For the initial validation of knowledge, all extracted information was emailed to two neurologist faculty members as domain experts, and they made additions and modifications.
The defined terms were organized using a top-down method, starting from the most general to more detailed concepts. Common concepts were defined as classes and arranged in a hierarchical order by using the “is-a” relationship.
Relations were represented by properties in Web Ontology Language (OWL) [20]. The SMTSO was developed by linking the ontology concepts.
To support the interoperability and sharing capabilities of STMSO, this ontology was designed as a sub-ontology of the Basic Formal Ontology (BFO) and the Ontology for General Medical Sciences (OGMS) top-level universal ontologies [21]. The BFO is an upper-level ontology designed to support information retrieval, analysis, and integration. BFO enables a realistic approach to ontology modeling, in which the classes in ontologies are universal categories of objects that represent things and processes [22]. OGMS is an ontology for the representation of diseases, signs, symptoms, clinical processes, diagnosis, treatment, and outcomes [21]. We used similar previous articles [13,23] to map the concepts into the BFO and OGMS.
Ontology development tools and languages were employed to implement the ontology. Specifically, the ontology was implemented on the researchers’ computer using the Protégé 5.0.0 ontology editor in OWL format [20]. Protégé is a free and open-source ontology editing tool developed by the Stanford Center for Biomedical Informatics Research. In this step, the defined classes and properties were implemented in Protégé 5. Figure 2 presents details. The defined rules were also implemented into the Semantic Web Rule Language (SWRL) tab in Protégé. The STMSO was uploaded to the National Center for Biomedical Ontology’s BioPortal (NC-BO’s BioPortal). This web portal supports a uniform mechanism to access biomedical terminologies and ontologies.
Design evaluation refers to the verification and validation of the ontology, including an assessment of the ontology’s consistency, and completeness. Consistency means that ontology does not encompass any contradictions [25], while completeness measures whether the domain of interest is adequately covered [26].
We used an open-source semantic reasoner, Pellet [27], with Protégé to verify the consistency of the ontology model during the ontology creation process. We also evaluated the ontology content against the defined list of CQs to ensure that it answered them. To assess the capability of the developed STMSO to answer the CQs, each CQ was represented by Simple Protocol and Rdf Query Language(SPARQL) queries to retrieve data from the ontology.
In this qualitative approach, the quality of the ontology was judged on the basis of expert opinion. Semi-structured interviews with domain experts were held to elicit their comments on the accuracy, clarity, and completeness of the ontology based on their knowledge and experience in this specialized field.
Accuracy is a criterion that evaluates whether the axioms of an ontology are consistent with domain knowledge [28], while clarity refers to the effectiveness of an ontology in conveying the intended meaning of defined terms [26]. The evaluation was conducted through in-person sessions during participants’ working hours that lasted for 35 to 45 minutes. The ontology was implemented on the researchers’ computer, and the domain experts (including two neurologist researchers, an experienced physiotherapist, and a consultant) were asked to manually review all entities (concepts and relationships) of the proposed ontology one by one. We then asked some open-ended questions to understand whether there were (1) irrelevant terms, (2) any entities missing (and, if so, which should be added), and (3) whether the structure, labels, and definitions of each entity were coherent.
It was established that the STMSO should provide a reference model for the representation of knowledge in the domain of symptomatic treatment focused on the specific scope of MS. The CQs included questions such as:
(1) Is it possible to search the ontology for pharmacological and non-pharmacological treatments for each symptom?
(2) Is it possible to search the ontology for suitable drug contraindications based on the patient’s status?
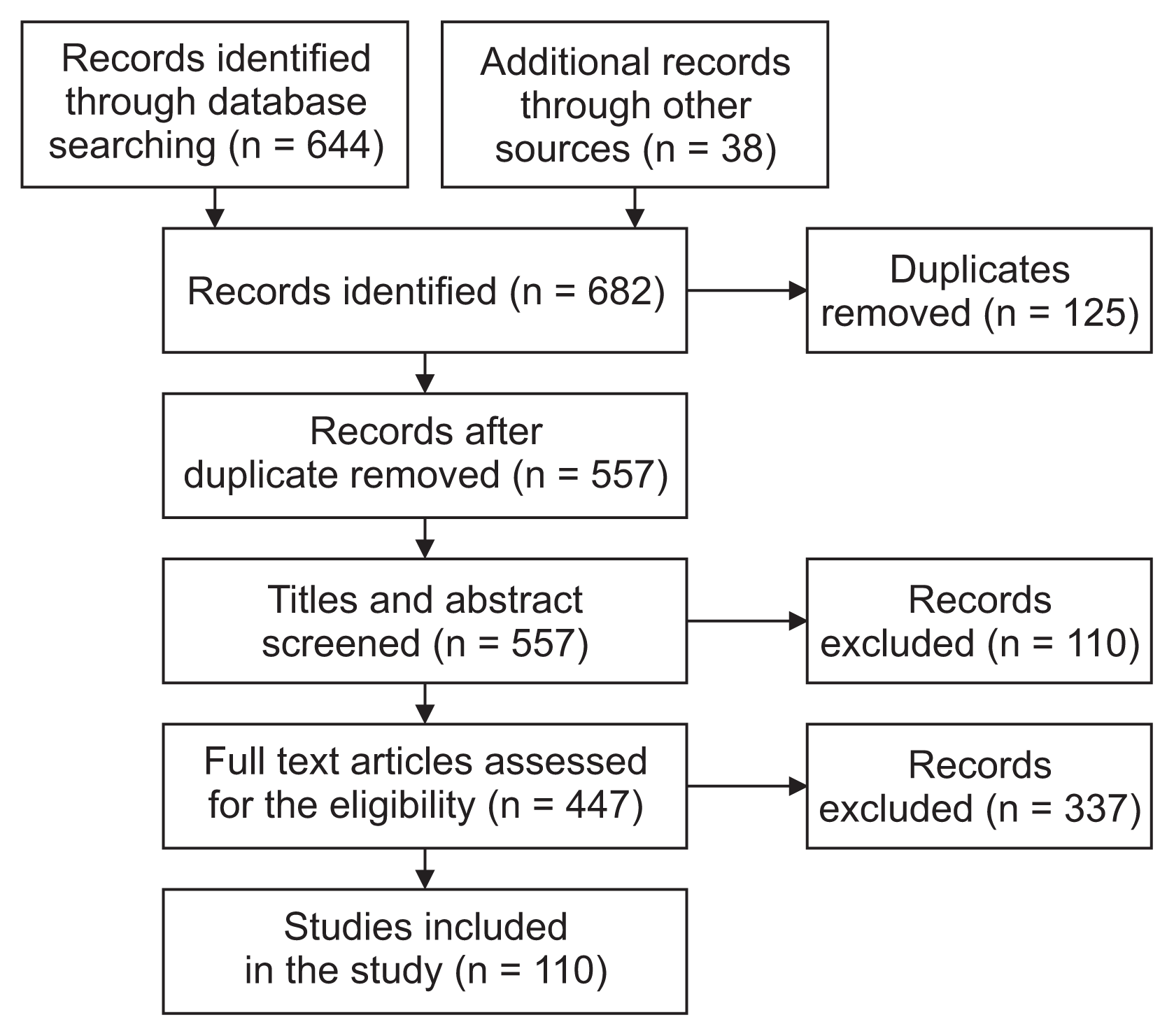
The systematic review identified 664 articles in the initial database search. Additional records were also added from other sources (n = 38), yielding a total of 682 records. After the removal of duplicates (n = 125), 557 articles remained for the initial screening of titles and abstracts. They were checked for eligibility, and 447 articles were excluded. Finally, a total of 110 full-text articles met the inclusion criteria for the current systematic review (Figure 3). First, we classified all articles based on symptoms. Articles were then read one by one, and data were extracted based on the checklist. A short selection of this checklist for spasticity, captured from literature is shown in Table 1. The detailed results of the checklist are shown in Supplement A.
We organized the rules into two categories: drug prescribing rules and other rules. Drug prescribing rules included contraindications for prescribing drugs due to a patient’s specific condition, such as age, pregnancy status, and the presence of an underlying disease. Other rules included the relationship between the patient’s disability status and the type of exercise and rules for distinguishing between a symptom and side effect of disease-modifying drugs. Detailed information about the rules is presented in Table 2. In this step, rules were defined (input) into the ontology. We defined 139 rules to customize a specific treatment plan using the SWRL editor plugin in Protégé. Table 2 shows some examples of rules from two rule categories and their triggering conditions. A full list of rules is also presented in Supplement B.
Examples of SWRL rules
First, the data identified by experts and the literature review as relevant for the symptomatic treatment of MS were classified into five groups, described in the ontology as five general classes (“patient,” “symptoms,” “pharmacological treatment,” “treatment plan,” and “measurement index”). A class hierarchy tree was then built according to the subsumption relationship (is-a-superclass-of, the converse of is-a-subclass-of) between classes. The STMSO incorporated a class count of 626, with a depth of two to six levels.
In this study, properties were mainly defined to connect symptom classes to treatment classes. The “is-a” relationship was used to provide the main hierarchy structure. The STMSO had 40 object properties. Figure 2 outlines some classes and object properties employed in the STMSO.
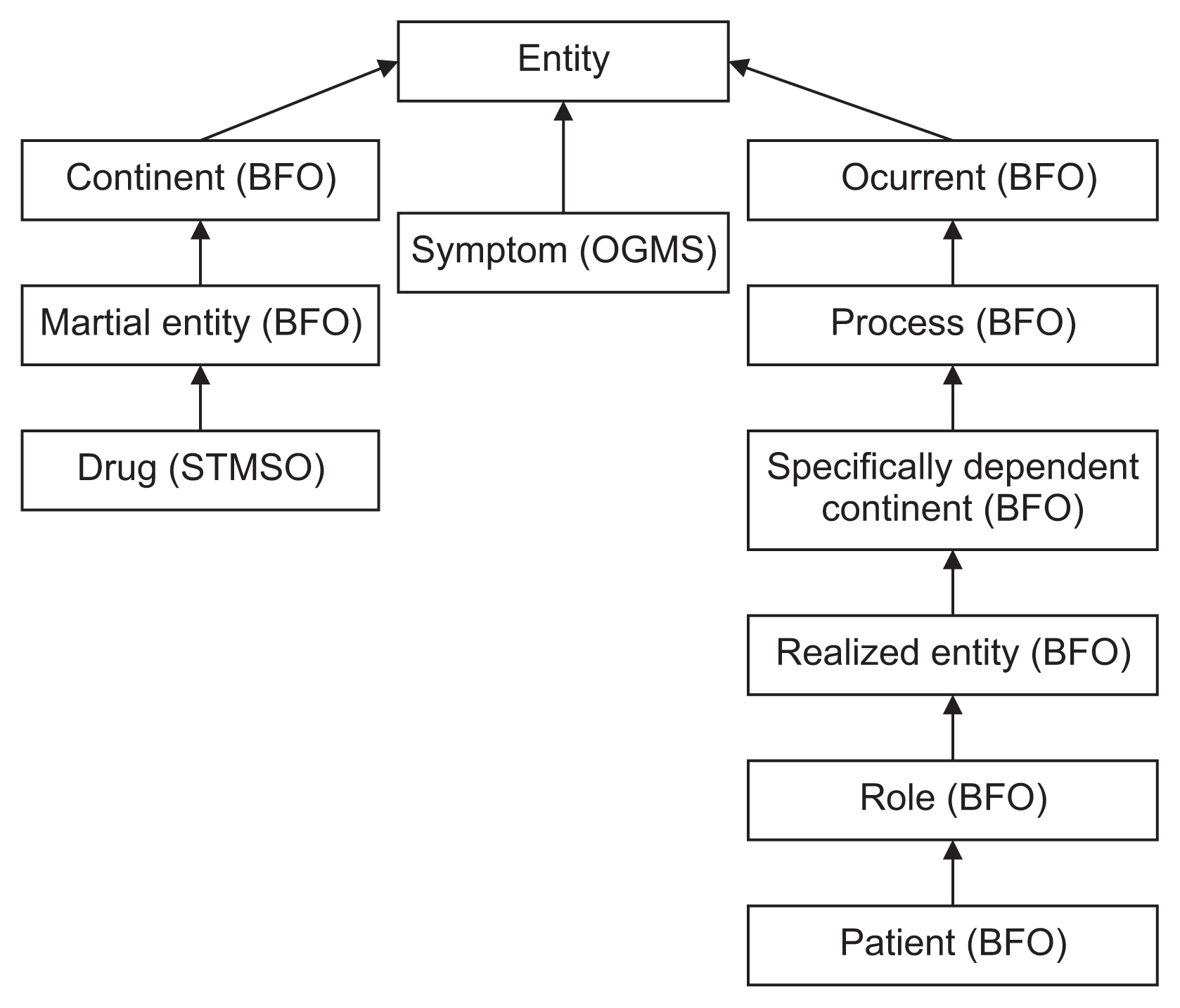
The main classes of the STMSO were implemented under the most suitable upper-level BFO and OGMS universals according to the semantics of these classes. For example, the STMSO’s pharmacological treatment class was implemented as a subclass of the BFO’s material entity.
In Figure 4, we present the asserted upper-level hierarchy of the STMSO, which shows how the top-level domainspecific classes are classified under the OGMS classes.
Two sets of ontologies were developed. After the evaluation of the first version in May 2021, the modified version was implemented 4 months later in September 2021. The revised ontology, including 626 classes, 40 object properties, and 139 rules, was implemented. The main components of STMSO are presented in Figure 2. The STMSO is publicly available for download through NCBO’s BioPortal at (https://bioportal.bioontology.org/ontologies/STMSO).
The evaluation using the Pellet reasoner showed that the STMSO was free of discrepancies, inconsistencies, and unsatisfactory classes. In the evaluation according to CQs, all questions can be answered from knowledge in the STMSO, indicating that the completeness standard was met. Table 3 lists a complementary and more specific list of CQs than the previously listed CQs in the requirement specification section. The CQs were represented as queries over the STMSO by using SPARQL queries.
Competency questions and corresponding axioms in the ontology
The accuracy of the STMSO was confirmed, as all experts said that no irrelevant terms were used. Moreover, the STMSO was based on knowledge derived from the most recent and reliable articles and clinical bulletins under the guidance of domain experts. Furthermore, during the entire development process, our domain experts were regularly consulted to verify the ontology’s correctness.
In terms of clarity, the physiotherapist said that it would be better to replace the term “spasticity” with “spasm.” All three other experts validated the collected terms and descriptions and confirmed their clarity, with no additional recommendations. The clarity of the STMSO was also achieved by assigning non-ambiguous labels or descriptions to each class, using “rdfs:label,” “rdfs:comment,” or “skos:definitions.” In terms of completeness, the results of the interview elicited 34 additional concepts that were added as new classes in the ontology. Examples of new classes that were added to the STMSO are listed in Table 4.
Some new added concepts and their definitions
In this study, we developed and evaluated a specific ontology entitled “STMSO” as a framework to improve the organization of knowledge about the symptomatic treatment of MS. The proposed ontology has good coverage of concepts related to MS symptomatic treatment. It has 626 classes regarding symptoms, treatments, and patient conditions. All treatment classes are related to each symptom through 40 object properties. It also has 139 rules that represent the challenges and complexities of symptomatic treatment. The evaluation results ensured the quality of the STMSO including accuracy, clarity, completeness, and consistency.
Semi-automatic approaches to knowledge extraction have been developed to build ontologies from texts. For example, Dostal et al. [29] used statistical and natural language processing techniques and automatic approaches to create domain ontologies, and we systematically collected and selected the relevant literature similar to Zhang et al. [30]. This approach ensures a credible and comprehensive knowledge base for the formulation of the ontology and makes the ontology complete and more coherent.
In our study, there are 13 classes for non-pharmacological treatments. This is in contrast to Malhotra et al. [14], who developed an ontology for MS that only included four classes for non-pharmacological treatment and did not have more detailed subclasses. Furthermore, our ontology provides a more organized classification of symptoms. For example, we placed all the visual problems in one class entitled “vision problems,” but Malhotra et al. [14] put “vision loss,” “ocular dysmetria,” and “nystagmus” into different classes.
The treatment class was an important class in our study that also existed in previous treatment ontologies in other domains [13,30]. We also included non-pharmacological treatment, with subclasses such as exercise therapy and music therapy that are similar to those in the diabetes mellitus treatment ontology [13].
The extracted rules in our study are different in terms of type and number from those in the study by El-Sappagh et al. [13]. We defined 139 rules in two categories, whereas they found 214 rules in four categories. Regarding the types of rules, we defined disease exercise interaction and medication rules that are similar to those of El-Sappagh et al. [13], but they also had education and lifestyle rules that are different from those in our study.
In line with former studies [13,30], the expert-based evaluation ensured the quality of the STMSO. Although this is a subjective evaluation approach, it is frequently considered a good validation process because it relies on the deep knowledge of external experts who can explore the quality of an ontology.
The STMSO has considerable potential as a domain ontology. It could transform clinical text data into machine-processable data and generate more insights on evidence-based treatment plans. These insights would help researchers and healthcare professionals better understand MS symptoms and effective treatments and eventually guide healthcare professionals to better symptomatic treatment, especially drug prescriptions, thereby ultimately improving the quality of care, patient safety, and satisfaction. We expect that the STMSO will be utilized to build CDSS to address specific challenges in physicians’ decision-making.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.4258/hir.2022.28.4.332.
Acknowledgments
The authors would like to thank Dr. Saba Naghavi, DR. Mohsen Kheradmand and Dr. Samira Ghasemi for their efforts in this work.
References
1. Daltrozzo T, Hapfelmeier A, Donnachie E, Schneider A, Hemmer B. A systematic assessment of prevalence, incidence and regional distribution of multiple sclerosis in Bavaria from 2006 to 2015. Front Neurol. 2018; 9:871.
https://doi.org/10.3389/fneur.2018.00871
.
2. Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of Multiple Sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014; 83(11):1022–4.
https://doi.org/10.1212/wnl.0000000000000768
.
3. Rommer PS, Eichstadt K, Ellenberger D, Flachenecker P, Friede T, Haas J, et al. Symptomatology and symptomatic treatment in multiple sclerosis: results from a nationwide MS registry. Mult Scler. 2019; 25(12):1641–52.
https://doi.org/10.1177/1352458518799580
.
4. Willems LM, Vriezekolk JE, Schouffoer AA, Poole JL, Stamm TA, Bostrom C, et al. Effectiveness of non-pharmacologic interventions in systemic sclerosis: a systematic review. Arthritis Care Res (Hoboken). 2015; 67(10):1426–39.
https://doi.org/10.1016/j.jns.2017.08.012
.
5. Miller P, Soundy A. The pharmacological and non-pharmacological interventions for the management of fatigue related multiple sclerosis. J Neurol Sci. 2017; 381:41–54.
6. Ng P, Murray S, Hayes SM. Clinical decision-making in multiple sclerosis: challenges reported internationally with emerging treatment complexity. Mult Scler Relat Disord. 2015; 4(4):320–8.
https://doi.org/10.1016/j.msard.2015.05.008
.
7. de Sa JC, Airas L, Bartholome E, Grigoriadis N, Mattle H, Oreja-Guevara C, et al. Symptomatic therapy in multiple sclerosis: a review for a multimodal approach in clinical practice. Ther Adv Neurol Disord. 2011; 4(3):139–68.
https://doi.org/10.1177/1756285611403646
.
8. Linker RA, Chan A. Navigating choice in multiple sclerosis management. Neurol Res Pract. 2019; 1:5.
https://doi.org/10.1186/s42466-019-0005-5
.
9. Maedche A, Staab S. Ontology learning for the semantic web. IEEE Intell Syst. 2001; 16(2):72–9.
https://doi.org/10.1109/5254.920602
.
10. Hadzic M, Chen M, Dillon TS. Towards the mental health ontology. In : Proceedings of 2008 IEEE International Conference on Bioinformatics and Biomedicine; 2008 Nov 3–5; Philadelphia, PA. p. 284–8.
https://doi.org/10.1109/BIBM.2008.59
.
11. Dissanayake PI, Colicchio TK, Cimino JJ. Using clinical reasoning ontologies to make smarter clinical decision support systems: a systematic review and data synthesis. J Am Med Inform Assoc. 2020; 27(1):159–74.
https://doi.org/10.1093/jamia/ocz169
.
12. Shen Y, Colloc J, Jacquet-Andrieu A, Guo Z, Liu Y. Constructing ontology-based cancer treatment decision support system with case-based reasoning. Qiu M, editor. Smart computing and communication. Cham, Switzerland: Springer;2017. p. 278–88.
https://doi.org/10.1007/978-3-319-73830-7_28
.
13. El-Sappagh S, Kwak D, Ali F, Kwak KS. DMTO: a realistic ontology for standard diabetes mellitus treatment. J Biomed Semantics. 2018; 9(1):8.
https://doi.org/10.1186/s13326-018-0176-y
.
14. Malhotra A, Gundel M, Rajput AM, Mevissen HT, Saiz A, Pastor X, et al. Knowledge retrieval from PubMed abstracts and electronic medical records with the Multiple Sclerosis Ontology. PLoS One. 2015; 10(2):e0116718.
https://doi.org/10.1371/journal.pone.0116718
.
15. Jensen M, Cox AP, Chaudhry N, Ng M, Sule D, Duncan W, et al. The neurological disease ontology. J Biomed Semantics. 2013; 4(1):42.
https://doi.org/10.1186/2041-1480-4-42
.
16. Jensen M, Cox AP, Ray P, Teter BE, Weinstock-Guttman B, Ruttenberg A, et al. An ontological representation and analysis of patient-reported and clinical outcomes for multiple sclerosis. In : Proceedings of the 5th International Conference on Biomedical Ontology (ICBO); 2014 Oct 8–9; Houston, TX. p. 52–5.
17. Noy NF, McGuinness DL. Ontology development 101 a guide to creating your first ontology. Stanford (CA): Knowledge Systems Laboratory, Stanford University;2001.
18. Schulz S, Grewe N, Rohl J, Schober D, Boeker M, Jansen L. Guideline on developing good ontologies in the biomedical domain with description logics [Internet]. Rostock, Germany: Universitat Rostock;2012. [cited at 2022 Oct 10]. Available from: https://www.iph.uni-rostock.de/forschung/homepage-goodod/guideline/
.
19. US Food and Drug Administration. Medication Guides [Internet]. Silver Spring (MD): Food and Drug Administration;c2022. [cited at 2022 Oct 10]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/medication-guides
.
20. Motik B, Patel-Schneider PF, Parsia B, Bock C, Fokoue A, Haase P, et al. OWL 2 web ontology language: structural specification and functional-style syntax. W3C Recommendation. 2009; 27(65):159.
21. Elmhadhbi L, Karray MH, Archimede B. Toward the use of upper-level ontologies for semantically interoperable systems: an emergency management use case. Popplewell K, Thoben KD, Knothe T, Poler R, editors. Enterprise interoperability VIII. Cham, Switzerland: Springer;2019. p. 131–40.
https://doi.org/10.1007/978-3-030-13693-2_11
.
22. Arp R, Smith B, Spear AD. Building ontologies with basic formal ontology. Cambridge (MA): MIT Press;2015.
23. El-Sappagh S, Ali F. DDO: a diabetes mellitus diagnosis ontology. Applied Informatics. 2016; 3:5.
https://doi.org/10.1186/s40535-016-0021-2
.
24. Obrst L, Ceusters W, Mani I, Ray S, Smith B. The evaluation of ontologies. Baker CJ, Cheung KH, editors. Semantic web. Boston (MA): Springer;2007. p. 139–58.
https://doi.org/10.1007/978-0-387-48438-9_8
.
25. Raad J, Cruz C. A survey on ontology evaluation methods. In : Proceedings of the International Conference on Knowledge Engineering and Ontology Development, part of the 7th International Joint Conference on Knowledge Discovery, Knowledge Engineering and Knowledge Management (IC3K); 2015 Nov 12–14; Lisbon, Portugal. p. 179–86.
26. Amith M, He Z, Bian J, Lossio-Ventura JA, Tao C. Assessing the practice of biomedical ontology evaluation: gaps and opportunities. J Biomed Inform. 2018; 80:1–13.
https://doi.org/10.1016/j.jbi.2018.02.010
.

27. Sirin E, Parsia B, Grau BC, Kalyanpur A, Katz Y. Pellet: a practical OWL-DL reasoner. J Web Semant. 2007; 5(2):51–3.
https://doi.org/10.1016/j.websem.2007.03.004
.

28. Pak J, Zhou L. A framework for ontology evaluation. Sharman R, Rao HR, Raghu TS, editors. Workshop on e-business. Heidelberg, Germany: Springer;2009. p. 10–8.
https://doi.org/10.1007/978-3-642-17449-0_2
.

29. Dostal M, Jezek K. Automatic keyphrase extraction based on NLP and statistical method. [place unknown]: DATESO2011;2011. [cited at Oct 10]. Available from: http://ceur-ws.org/Vol-706/poster13.pdf
.
30. Zhang Z, Yu P, Chang HC, Lau SK, Tao C, Wang N, et al. Developing an ontology for representing the domain knowledge specific to non-pharmacological treatment for agitation in dementia. Alzheimers Dement (N Y). 2020; 6(1):e12061.
https://doi.org/10.1002/trc2.12061
.





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download