Laparoscopic surgery has several advantages over laparotomy in patients who require hepatectomy. It is a minimally invasive procedure that results in less bleeding during surgery, rapid recovery, and reduced postoperative pain [
1].
To ensure visibility during laparoscopic surgery, pneumoperitoneum is establlished by injecting air or gas intraperitoneally under controlled pressure. The most commonly used gas is carbon dioxide due to its high diffusion rate [
2]. The physiologic effects of pneumoperitoneum include reduced cardiac output and increased systemic vascular resistance. CO
2-induced pneumoperitoneum causes additional effects, such as respiratory acidosis and deep vein thrombosis [
3]. CO
2 embolism occurs when CO
2 is injected into the vascular system. In animal and human studies using transesophageal echocardiography (TEE), the incidence of CO
2 embolism during laparoscopic procedures was 65–100%, and though these embolisms resulted in respiratory and cardiovascular compromise, they resolved spontaneously. Though the incidence of significant CO
2 embolisms that cause detrimental outcomes is rare (0.001% to 0.59%) [
2], they can manifest as a life-threatening complication during laparoscopic hepatectomy [
4]. The incidence of CO
2 embolism during general laparoscopic surgery is 0.15% but rises to 1.2–4.6% during laparoscopic hepatectomy, which is approximately ten times higher than that observed during general laparoscopic surgery [
4,
5].
We encountered a case of CO2 embolism due to portal vein rupture during laparoscopic surgery for hepatic cell carcinoma in a 70-year-old male. We detected the embolism early using perioperative TEE and responded appropriately. Herein, we report this case.
CASE REPORT
Written informed consent was obtained from our patient for the publication of this report. Institutional Review Board approval number DFE21ORIO101, Daegu Fatima Hoapital.
A 70-year-old male (height 154.6 cm, weight 53.6 kg) was diagnosed with hepatic cell carcinoma and admitted for laparoscopic right colectomy posterior sectionectomy (right hepatic vein preserving). The patient had experienced a cerebral infarction at 67 years of age but he did not present with any significant sequelae. He also had a history of benign prostatic hyperplasia and major depressive disorder. Electrocardiography (ECG), chest computed tomography, pulmonary function test, transthoracic echocardiography (TTE) and laboratory examinations (complete blood count [CBC], partial and activated partial thromboplastin times, aspartate transaminase/alanine transaminase levels, blood urea nitrogen-to-creatinine ratio, glomerular filtration rate, sodium level, potassium level, protein level, albumin level and urinalysis), were performed.
Results of the CBC revealed hemoglobin and hematocrit levels at 11.8 g/dl and 36.9%, respectively. Chest computed tomography showed the presence of a calcified nodule (0.9 cm) at segment 7 of the liver, subsegmental atelectasis in the right middle and left upper lung lobes, and mild atherosclerotic calcification at the aorta. The patient’s left ventricular systolic function was normal (visual estimated ejection fraction 60%), with no regional wall motion abnormality. Impaired relaxation and increased intima-media thickness of the right common carotid artery were observed on TTE.
The patient was administered intramuscular glycopyrrolate (0.2 mg) and intravenous famotidine (20 mg) 30 min before surgery. Non-invasive blood pressure (BP) monitoring, ECG (lead II), pulse oximetry, end-tidal (Et) CO2 monitoring, and bispectral index (BIS) monitoring were performed. Before induction of anesthesia, vital signs were checked, and a BP of 211/94 mmHg (mean BP of 133 mmHg), heart rate (HR) of 60 beats/min with regular sinus rhythm, and peripheral oxygen saturation (SpO2) of 98% were noted.
For anesthesia induction, 100% oxygen (O2) was administered through a mask, and 1% lidocaine (50 mg), propofol (120 mg), and rocuronium (50 mg) were administered intravenously. Tracheal intubation was performed using a cuffed endotracheal tube with an internal diameter of 7.5 mm. Anesthesia was maintained at a BIS of 40–60 using 2 L/min of O2, 2 L/min of fresh air, 2.0 vol% of sevoflurane, and remifentanil 3.7 μg/kg/h to 7.5 μg/kg/h. The EtCO2 level was maintained in the range of 35–40 mmHg with a volume-controlled ventilator (tidal volume, 450 ml, respiratory rate 14 breaths/min, positive end-expiratory pressure 0 mmHg, inspiration-expiration ratio 1:2). Arterial BP (ABP) was maintained in the range 60–110 mmHg based on mean ABP (MAP). At the surgeon’s request, a low central venous pressure (CVP) of < 5 mmHg was maintained during the operation. Rocuronium was administered intravenously at a rate of 26 mg/h. Intravenous fluid was maintained at a rate of 200 ml/h with plasma solution.
Arterial cannulation was performed for continuous BP monitoring in the right radial artery, and a central venous catheter was placed in the right subclavian vein. The surgery was performed with the patient in the left semilateral decubitus position using the supraumbilical trocar insertion and CO2 intraperitoneal insufflation techniques. Pneumoperitoneum was maintained using a variable-flow insufflator with a pressure of 12 mmHg.
Two hours into the procedure, an ABP of 76/51 mmHg (MAP, 59 mmHg) and an HR of 84 beats/min were noted. The ABP was stable after administering 100 μg of phenylephrine bolus intravenously. At 3 h, an ABP of 74/52 mmHg (MAP, 59 mmHg) and an HR of 84 beats/min were noted, and 50 μg of phenylephrine was again administered intravenously by bolus.
After 4 h, the patient’s vital signs were stable, although a blood loss of 500 ml was noted. Therefore, 500 ml of 6% hydroxyethyl starch fluid was injected through the C-line over 1 h. Five hours into the procedure, the EtCO2 level suddenly increased from 35 mmHg to 55 mm Hg, ABP decreased from 106/63 mmHg (MAP, 77 mmHg) to 57/45 mmHg (MAP, 49 mmHg), SpO2 decreased from 97% to 45%, HR decreased from 97 beats/min to 84 beats/min, and CVP increased from 3 mmHg to 6 mmHg. Additionally, ECG showed a change in the regular sinus rhythm (SR) to SR with ST elevation. The BIS remained at 40–45. We informed the surgeon of the patient’s condition and started ventilation with 100% O2 and immediately administered 10 μg of epinephrine intravenously while injecting plasma solution at 1,000 ml/h. Subsequently, norepinephrine was administered intravenously, starting at 0.1 μg/kg/min and increased to a rate of 0.5 μg/kg/min. For ECG changes, nitroglycerin was administered intravenously at 0.6 μg/kg/min. Arterial blood gas analysis (ABGA) showed a pH of 7.119, partial pressure of CO2 (PaCO2) of 75.9 mmHg, partial pressure of O2 (PaO2) of 96 mmHg, bicarbonate (HCO3) level of 24.6 mM/L, and an arterial oxygen saturation (SaO2) of 94%. A check of vital signs after 5 min showed an ABP of 109/58 mmHg (MAP, 75 mmHg), an HR of 103 beats/min, and a CVP of 5 mmHg. The SpO2 was 81%, EtCO2 was 55 mmHg, and the BIS was 31. However, 5 min later the SpO2 changed to 100% and EtCO2 to 33 mmHg. The ST elevation on ECG disappeared and regular sinus rhythm was observed. The vital signs were stable for approximately 20 min, although EtCO2 changed to 60 mmHg, ABP to 73/48 mmHg (MAP, 56 mmHg), HR to 95 beats/min, CVP to 4 mmHg, SpO2 to 41%, and the BIS to 31 (from 45). At this time, the ST elevation was observed again on ECG. Accordingly, norepinephrine was increased to 0.75 μg/kg/min, and dobutamine was injected intravenously at a rate of 6.2 μg/kg/min. We maintained ventilation with 100% O2 and administered plasma solution at 1,000 ml/h intravenously.
ABGA showed a pH of 6.93, PaCO2 of 110.6 mmHg, PaO2 of 38 mmHg, an HCO3 level of 26.8 mM/L, and an SaO2 of 42%. We informed the surgeon of the patient’s condition, and the surgeon conveyed information regarding the portal vein laceration that had occurred during the procedure. As a gas embolism was strongly suspected, the bed was immediately changed to the head-down and right-up position to prevent neurological complications and retard the movement of CO2 to the left heart chambers. TEE was performed.
The intraoperative TEE findings showed that the septum between the right and left atria was open, which was undetectable on preoperative TTE. Air bubbles moved to the left atrium in the mid-esophageal bicaval view on TEE (
Supplementary Video 1,
Fig. 1). Additionally, in the mid-esophageal aortic valve short-axis view, air bubbles were observed in the aortic valve and the right-to-left shunt was identified using color Doppler imaging (
Supplementary Video 2,
Fig. 2). A large number of air bubbles were observed in the left atrium and ventricle, and the opening of the right and left atrial septa in the mid-esophageal four-chamber view (
Supplementary Video 3,
Fig. 3). The air bubbles in the left ventricle moved to the aorta in the mid-esophageal long axis view (
Supplementary Video 4,
Fig. 4).
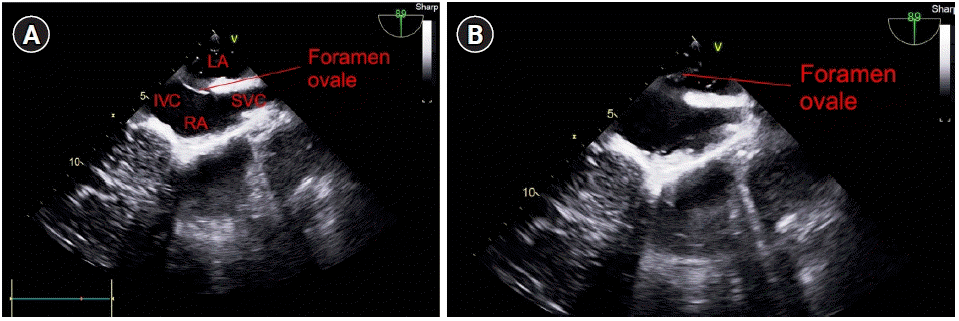 | Fig. 1.Mid-esophageal bicaval view of the transesophageal echocardiogram. (A) The septum between the right and left atria is observed to be closed, and (B) the septum between the right and left atria is observed to be open. LA: left atrium, IVC: inferior vena cava, SVC: superior vena cava, RA: right atrium. 
|
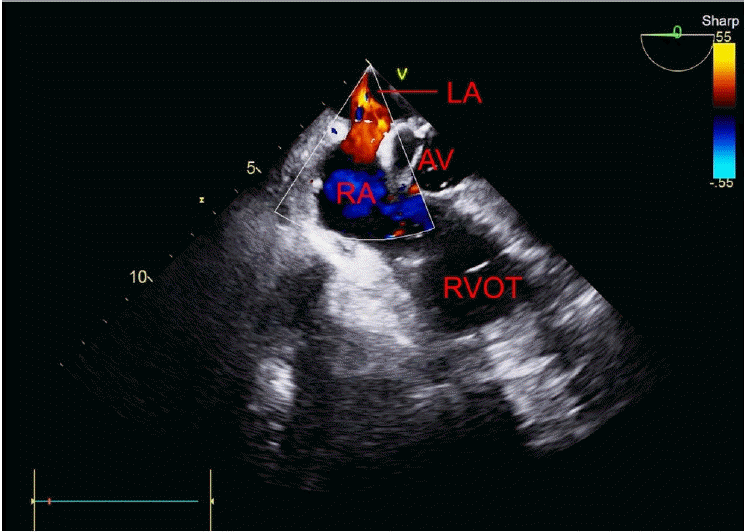 | Fig. 2.Mid-esophageal aortic valve short-axis view of the transesophageal echocardiogram with color Doppler shows blood flow (right-to-left shunt) between the right and left atrial septa. LA: left atrium, AV: aortic valve, RA: right atrium, RVOT: right ventricular outflow tract. 
|
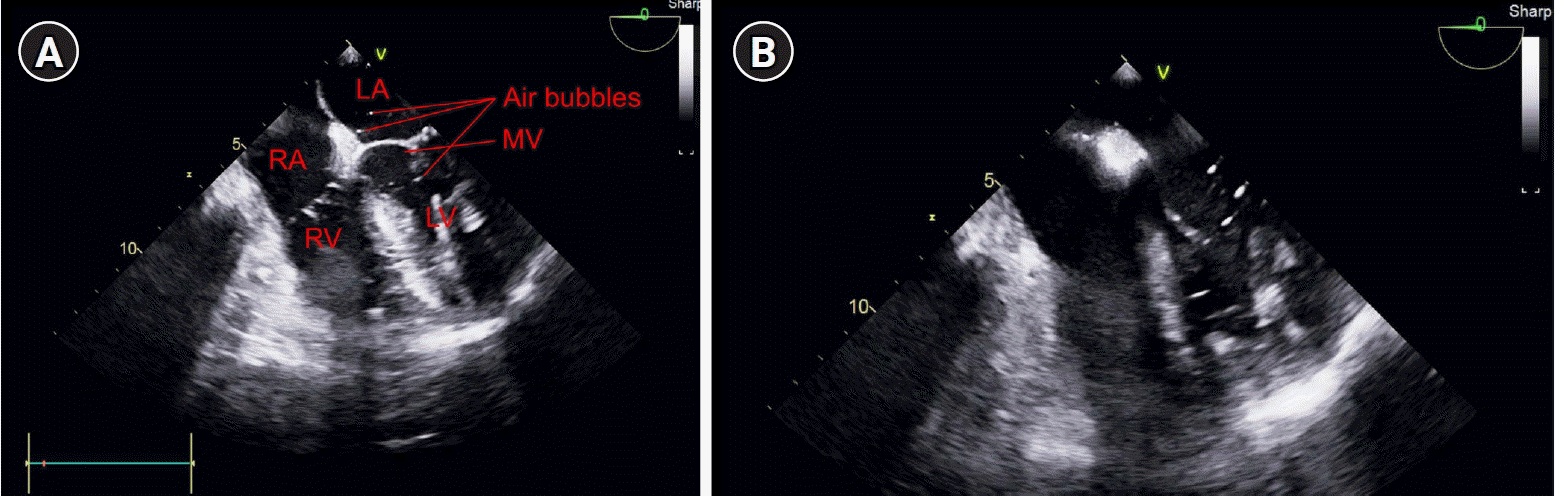 | Fig. 3.Mid-esophageal four-chamber view of the transesophageal echocardiogram. (A) During the left ventricle systolic period, the mitral valve is closed, and air bubbles are observed in the left atrium. (B) During the left ventricle diastolic period, the mitral valve is opened, and a large number of air bubbles are observed in the left ventricle. LA: left atrium, RA: right atrium, MV: mitral valve, RV: right ventricle, LV: left ventricle. 
|
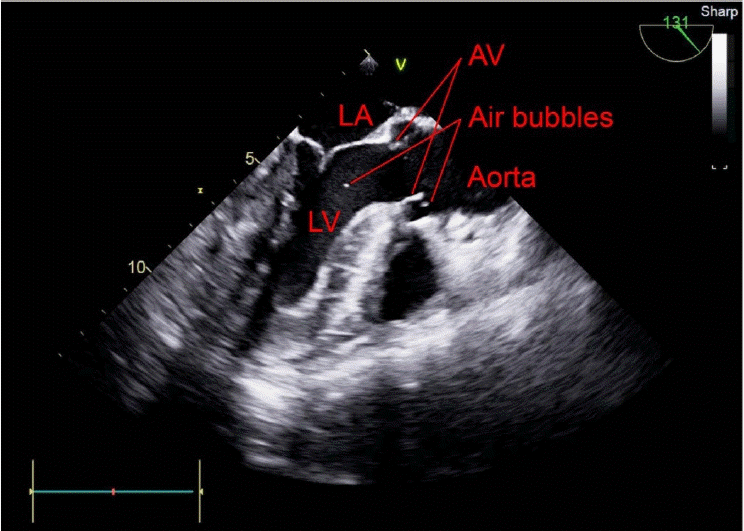 | Fig. 4.Mid-esophageal long axis view of the transesophageal echocardiogram shows the gas in the left ventricle passing through the aorta. LA: left atrium, AV: aortic valve, LV: left ventricle. 
|
After 30 min, the patient’s vital signs were checked and the following were noted: ABP, 107/74 mmHg (MAP 85 mmHg); HR, 91 beats/min; CVP, 4 mmHg; SpO2, 100%; EtCO2. 38 mmHg; and BIS, 41. On the ABGA, a pH of 7.241, PaCO2 of 57.0 mmHg, PaO2 of 141 mmHg, an HCO3 level of 23.1 mM/L, and an SaO2 of 99% were observed. Air bubbles were not observed on TEE, the right-to-left shunt of the atrial septum was not noted, and vital signs remained stable. The surgery was completed without any further complications.
The patient was administered sugammadex (200 mg) to reverse the effects of the neuromuscular blocking agents and extubation was performed. After confirming that our patient had recovered consciousness, he was transferred to the recovery room while an infusion of norepinephrine, dobutamine, and remifentanil was maintained. In the recovery room, the patient had an ABP of 105–141/40–59 mmHg (MAP, 61–86 mmHg) and an HR of 97–110 beats/min. The patient had a stable ECG that showed regular sinus rhythm and he had an alert mental status. ABGA showed a pH of 7.239, PaCO2 of 45.3 mmHg, PaO2 of 96.1 mmHg, HCO3 of 18.9 mM/L, and SaO2 of 96.1%. Hemoglobin and hematocrit levels of 10.7 g/dl and 33.7%, respectively, were checked using a CBC. TTE was conducted in the recovery room, confirming that there were no air bubbles in the heart and that the right and left atrial septa were closed.
After spending 1 h in the recovery room, the patient was transferred to the intensive care unit. Norepinephrine was maintained at 0.15 μg/kg/min, dobutamine at 5.6 μg/kg/min, and nitroglycerin at 0.6 μg/kg/min.
The patient was discharged from the hospital without cardiopulmonary or neurological sequelae.
In a post-surgery discussion with the surgeon, he informs us that there was a portal vein rupture approximately 5 min prior to the occurrence of the first symptom (
Fig. 5).
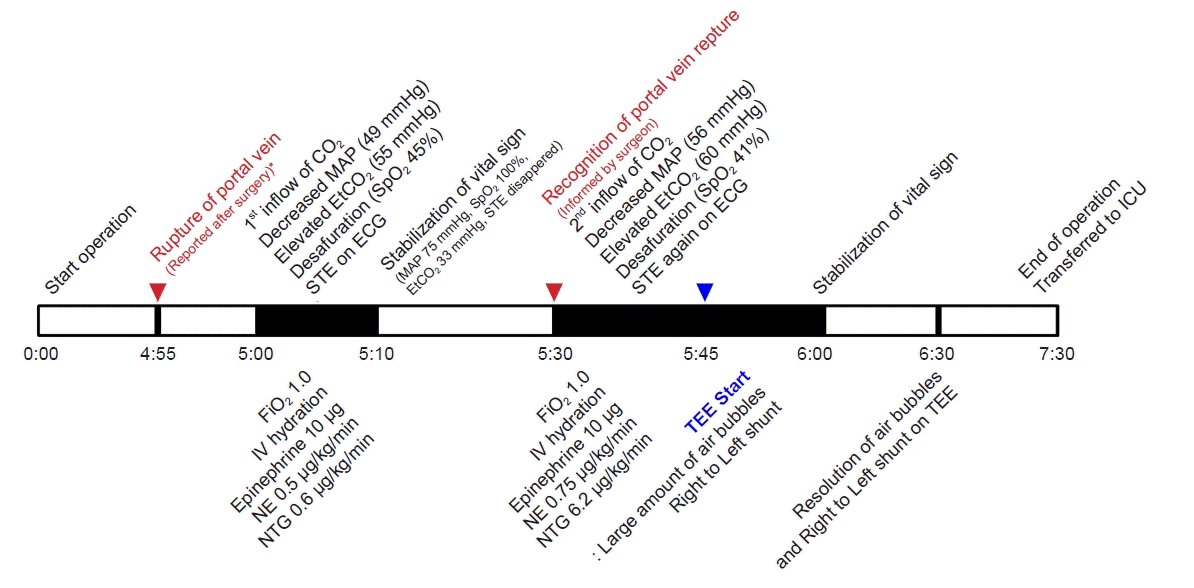 | Fig. 5.Timeline of the occurrence of CO2 embolism. MAP: mean arterial pressure, EtCO2: end-tidal carbon dioxide, SpO2: saturation of percutaneous oxygen, STE: ST elevation, ECG: electrocardiography, FiO2: fraction of inspired oxygen, IV: intravenous, NE: norepinephrine, NTG: nitroglycerin, TEE: transesophageal echocardiography, ICU: intensive care unit. *In a post-surgery discussion with the surgeon, he informs us that there was a portal vein rupture approximately 5 min prior to the occurrence of the first symptom. 
|
Go to :

DISCUSSION
Intraoperative significant CO
2 embolism in laparoscopic hepatic surgery is a rare, severe complication and can occur when a blood vessel with a pressure lower than the intra-abdominal pressure is opened [
6]. In venous embolism, emboli pass to the right heart or pulmonary artery/central vein. Arterial air embolism is caused by the introduction of air to the left heart chamber through paradoxical air embolism due to intracardiac shunt, cardiac surgery, or direct inflow into the artery, such as decompression barotraumas or penetrating trauma [
7]. When CO
2 enters the circulatory system, a CO
2 embolism moves to the inferior vena cava through the right atrium and right ventricle and enters the pulmonary artery or pulmonary circulatory system. This mechanism is called a “gas lock” [
8,
9]. During hepatic laparoscopy, peritoneal insufflation and gall bladder or liver dissection occur mainly in two stages and are normally associated with venous air embolism [
10].
CO
2 in the circulatory system is eliminated through three main mechanisms: diffusion, conversion to carbonic acid by binding to water, or binding to a protein. When a CO
2 embolism enters pulmonary circulation, elimination is achieved by reabsorption to an adjacent structure. Air trapped in the pulmonary circulatory system until the end of this process stops pulmonary blood flow, leading to an increase in ventilatory dead space that causes acute hypoxemia and hypercapnia. An acute increase in pulmonary vascular resistance occurs with acute right atrium strain, leading to right heart failure and possible circulatory collapse. Moreover, accumulation of air in the left ventricle prevents adequate diastolic filling and air is pumped into the coronary arteries during systole, impeding coronary perfusion [
11].
Clinical symptoms of CO2 embolism include rapid decrease or increase in EtCO2, elevated pulmonary arterial pressure, marked hypotension, dysrhythmias, hypoxia, cyanosis, pulmonary edema, and precordial or esophageal auscultation of “mill” murmur.
As mentioned above, when a CO
2 embolism occurs, either a sudden decrease or sudden increase in the EtCO
2 level is possible. The decrease in EtCO
2 is due to the decrease in significant perfusion resulting from a decrease in pulmonary blood flow as the inflow of CO
2 enters the pulmonary circulation and “gas locking” occurs. On the other hand, the EtCO
2 increases as the concentration of CO
2 in the blood increases as this gas dissolves rapidly in the bloodstream. In the case of general pulmonary gas embolism, as the pulmonary vasculature is obstructed, pulmonary perfusion decreases and dead space increases, resulting in a decrease in EtCO
2. However, when paradoxical embolism occurs due to patent foramen ovale, the inflow of CO
2 is pumped from the left ventricle, moves to the circulatory system or coronary artery, dissolves in the blood, and the CO
2 concentration in the blood increases, causing an elevation in EtCO
2 levels. In both sets of circumstances, the PaCO
2 increases as CO
2 flows directly into the bloodstream [
9].
In the case of our patient, both EtCO2 and PaCO2 were observed to increase simultaneously. This would happen because CO2 is continuously absorbed into the blood due to pneumoperitoneum, and a large amount of CO2, introduced through the portal vein rupture, would move through the PFO and dissolve rapidly through the entire circulatory system. EtCO2 and PaCO2 levels would then increase simultaneously.
However, CO
2 embolism generally presents with non-specific symptoms and may be overlooked. Air bubbles detected through perioperative TEE are often the only clinical manifestation of the condition. In this case, air bubbles were observed in the right atrium, right ventricle, pulmonary artery, and inferior vena cava on TEE. Therefore, although it is not routinely used in laparoscopic surgery, TEE is a sensitive tool for diagnosing CO
2 gas embolism, and even small emboli can be detected [
9,
12].
Early recognition is crucial in the treatment of CO
2 embolism. However, even if CO
2 embolism is suspected during laparoscopy, it is difficult to directly capture the embolism through imaging as it takes several minutes to set up the TEE equipment and the highly soluble CO
2 rapidly diffuses into the bloodstream. Thus, EtCO
2 monitoring is critical for the early detection of venous CO
2 embolism [
13]. If CO
2 embolism is suspected, prompt action should be taken. Possible treatments for this type of embolism include contracting the pneumoperitoneum, inducing hyperventilation, moving the patient into the Trendelenburg position, aspiration (using a central venous catheter), intravenous fluid administration, and hyperbaric oxygen therapy [
2].
In our case, CO2 embolism occurred due to a laceration of the portal vein. Considering the sudden rise in EtCO2 and changes in vital signs, we suspected that there were two inflows of CO2. When the second inflow occurred, close communication between the surgeon and anesthesiologist enabled prompt identification of a suspected CO2 gas embolism and rapid use of TEE to identify the CO2 bubbles. Moreover, bubbles were observed in the left atrium and ventricle, as well as the right atrium. Using color Doppler imaging, we identified that right-to-left shunt occurred through the septum of the right and left atria.
At the first inflow, we reported to the surgeon that the patient’s vital signs were unstable, but all vital signs stabilized within 5 min. The surgeon did not tell us that the portal vein had ruptured. When the second inflow occurred and we reported it, we were informed about the portal vein rupture. Therefore, CO2 embolism was strongly suspected when the second event occurred. As no specific cardiac abnormalities such as PFO were observed on the TTE performed for preoperative evaluation, it was difficult to suspect coronary air occlusion due to paradoxical embolism, and stress-induced cardiomyopathy was more strongly suspected.
As the patient had a PFO, “gas locking,” which occurred in the right atrium and pulmonary artery, increased pulmonary arterial and right atrial pressure, forcing the foramen ovale open. In addition, ST elevation was observed, possibly due to the pumping of gas from the left ventricle to the coronary arteries. When gas embolism occurs during positive pressure ventilation in patients with a PFO, gas escapes from the right atrium into the left atrium through the opened foramen ovale, resulting in paradoxical gas embolism [
14].
In our patient, although preoperative TTE was performed, a PFO was not diagnosed. The PFO would not have been identified on TTE because the foramen ovale remained closed by the left and right atrial pressure gradients, even with incomplete closure [
15].
In this case report, we aimed to emphasize the prevalence of CO2 embolism and the necessity of communication between surgeons and anesthesiologists in the operating room to identify pivotal moments of vascular exposure. Through effective communication, we can quickly recognize potential and fatal complications during laparoscopic hepatic surgery. Moreover, given the limitations, perioperative TEE is actively recommended if any of the following situations occur: an increase in abdominal pressure due to pneumoperitoneum, changes in vital signs, and changes in the patient’s condition due to organ damage. Morbidity and mortality due to CO2 embolism can be significantly reduced if the condition is recognized early during laparoscopic hepatic surgery, and the symptoms are promptly diagnosed and responded to when an event occurs. In conclusion, care should be taken with patients undergoing laparoscopic surgery involving large veins to prevent catastrophic CO2 embolism.
Go to :









 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download