Abstract
Background
Pulmonary hypertension in pregnancy is rare and leads to high maternal morbidity and mortality.
Case
A 27-year-old parturient woman with a 31-week gestational age underwent cesarean delivery under combined spinal-epidural anesthesia. She had systemic lupus erythematosus associated with severe pulmonary arterial hypertension. The operation was done in the cardiac theatre along with meticulous invasive monitoring. Insertion of femoral artery and femoral vein catheters for veno-arterial extracorporeal membrane oxygenation was done before delivery as preparation for the potential emergency of a life-threatening form of decompensated cardiac failure. During the delivery, the patient suddenly developed increased pulmonary arterial pressure. This was controlled by the continuous infusion of intravenous milrinone.
Pulmonary hypertension (PH) is the condition of pulmonary vasoconstriction. It is diagnosed by right heart catheterization with a demonstrated mean pulmonary arterial pressure (mPAP) of 20 mmHg or greater when measured at rest by the Sixth World Symposium on Pulmonary Hypertension in 2018 [1] or greater than 25 mmHg at rest as per the guidelines issued by the European Society of Cardiology (ESC)/European Respiratory Society (ERS) in 2015 [2]. The World Health Organization classifies PH into 5 groups. They are: (1) pulmonary arterial hypertension (PAH); (2) PH due to left heart disease; (3) PH due to chronic lung disease, hypoxia, or both; (4) PH due to chronic and pulmonary vascular thromboembolic disease; and (5) PH due to unclear or multifactorial causes [3]. PH caused by systemic lupus erythematosus (SLE) is classified as Group 1. The effects of SLE cause imbalances between vasodilators (prostacyclin, prostaglandin I2) and vasoconstrictors mediators (endothelin-1 and, thromboxane A2). This generates more vasoconstrictors, leading to vasoconstriction and hypoxia in the lungs and overexpression of the hypoxia-inducible factor and erythropoietin. These effects subsequently produce an accumulation of inflammatory cells in the pulmonary arteries. The cells aggravate the vascular remodeling process and contribute to increases in pulmonary vascular resistance (PVR) and PAH [4]. Although the incidence of PH during pregnancy was found to be only 1.1:100,000, the maternal mortality rate is very high (25–56%) [5,6]. This is due to right-sided heart failure, pulmonary hypertensive crisis, pulmonary thromboembolism, or arrhythmias [7]. The physiological changes to the cardiovascular system during pregnancy, especially during the second and third trimesters, are an increased blood volume, increased cardiac output, and decreased PVR and systemic vascular resistance. These changes affect patients with PH during pregnancy. An increasing volume overload of the right ventricle (RV) leads to decompensation and heart failure. In early pregnancy, the guidelines recommend giving early advice to terminate the pregnancy [6]. Should the pregnancy be continued, a multidisciplinary team approach at a tertiary center ensures the best clinical outcomes. The authors present a report of success in providing anesthesia for the cesarean delivery of a parturient woman with SLE-associated PAH. The patient provided written consent for the publication of this report.
A 27-year-old pregnant woman was referred to our hospital. She was G2P1A0, with a 31-week gestational age (at March 2021). She weighed 60 kg and was 165 cm tall. Her underlying disease, SLE, was diagnosed at the age of 11 years (15 years before this admission). The diagnostic criteria related to SLE included autoimmune hemolytic anemia, lymphopenia, neutropenia, malar rash, oral ulcer, positive anti-double-stranded DNA, positive antinuclear antibody, and lupus nephritis with proteinuria. The woman was initially treated with prednisolone and hydroxychloroquine for 6 years until she achieved clinical remission with successful tapering off of steroids. Until the age of 18 years (9 years ago), the sole symptom of SLE was her becoming easily fatigued. In 2012, a transthoracic echocardiograph and a right heart catheterization with a pulmonary vasodilatation test confirmed the diagnosis of PAH. The condition did not respond to nitric oxide (mPAP, 42.5 mmHg; pulmonary arterial pressure [PAP], 50/38 mmHg; left ventricular ejection fraction, 68%; D-shaped left ventricle, right ventricular dilatation). Based on the woman’s clinical presentation, etiology, and disease severity, the patient received only supportive therapies with aspirin (81 mg) to prevent a thrombotic event. In 2019 (2 years before being referred to the hospital), she became pregnant. No abnormality was apparent during the pregnancy, and she gave vaginal delivery at the 38th gestational week. Subsequently, the progression of SLE and PAH remained consistently stable, with a New York Heart Association classification of I. Six months before being referred to the hospital, an SLE flare was detected by aggravated symptoms of progressive dyspnea, precipitated by 12 weeks of gestation. For 3 weeks, the patient had dyspnea even on ordinary exertion, and her New York Heart Association classification rose to III. The patient was admitted to the respiratory intensive care unit due to severe shortness of breath at the gestational age of 29 weeks.
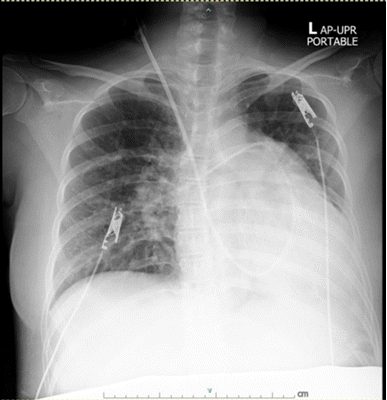
On admission, the woman’s current medications were prednisolone, azathioprine, hydroxychloroquine, aspirin, methyldopa, folic acid, iron combined with iodine, and calcium carbonate. Cardiovascular and respiratory assessments by a cardiologist and pulmonologist revealed that the patient’s symptoms had worsened from severe PAH and required targeted therapies. A transthoracic echocardiograph showed normal systolic function; a left ventricular ejection fraction of 58%; concentric left ventricular hypertrophy; abnormal septal motion due to volume and pressure loading in the RV; dilation of the right atrium and RV with RV hypertrophy; a normal RV systolic function (assessed by a tricuspid annular plane systolic excursion of 19 mm); marked pulmonary artery dilatation; moderate tricuspid and pulmonic regurgitation with PAH; a PAP of 59/20 mmHg; an mPAP of 45 mmHg; and no intracardiac shunt. A pulmonary artery (PA) catheter was placed on hospital day 4 to guide treatment. The parameter values detected by the catheter were as follows: PAP, 68/17 mmHg; mPAP, 37 mmHg; pulmonary capillary wedge pressure, 13 mmHg; cardiac output, 5.9 L/min; cardiac index, 3.68 L/min/m2; and stroke volume, 76 ml/beat (Table 1). Meanwhile, hemodynamic stability was achieved: her blood pressure was 138/85 mmHg; the pulse rate was 80 beats/min; and her respiratory rate was 18 times/min. A complete blood count showed hemodilutional anemia with hematocrit of 26.4%; other blood-chemistry and coagulation values were within normal limits. In addition, a chest X-ray revealed a marked increase in the size of her heart, a prominent pulmonary trunk with pulmonary edema, proper positioning of the tip of the PA catheter, and moderate pleural effusion (Fig. 1).
The patient was informed that it was considered necessary to control her PAP by administering pulmonary vasodilators. She was also warned that their use might have a negative impact on the fetus. After giving consideration, the patient consented to the treatment. Subsequently, 40 mg of sildenafil was taken every 8 h and iloprost (Ventavis®, Bayer, Germany ) was inhaled every 3 h using a standard delivery technique. Adjuvant therapies were oxygen administration and restricted fluid intake by balancing input and output. The intravenous steroid dexamethasone was given to accelerate fetal lung maturation. The patient developed premature uterine contractions on hospital day 9; these were suppressed through the administration of tocolytic oral nifedipine (5 mg) every 6 h.
Given that the case involved a high-risk pregnancy, a multidisciplinary team (obstetricians, cardiologists, pulmonologists, cardiac surgeons, and obstetric anesthesiologists) was established on hospital day 10 to optimize the timing and location of the delivery. All members agreed with the scheduling of an elective cesarean delivery in the cardiac operating theatre during the 31st gestational week with preparing for the veno-arterial extracorporeal membrane oxygenation (ECMO) circuit by insertion the introducer sheath before cesarean delivery. The authors and the multidisciplinary team prepared the blood components for intraoperative bleeding (4 units of packed red cells, 6 units of platelet concentrate, and 1,000 ml of fresh frozen plasma). Iloprost inhaler, sildenafil, and hydrocortisone were used as preoperative medications on the day of surgery.
In the cardiac operating theatre, standard monitors were used (5-lead electrocardiogram, urine output, temperature, and pulse oximetry). The patient’s vital signs were as follows: heart rate, 75 beats/min; blood pressure, 153/93 mmHg; respiratory rate, 20 breaths/min; body temperature, 36.8℃; and oxygen saturation, 96%. Oxygen was given at 3 L/min via nasal cannula. Before starting the anesthesia, a left-radial artery catheter was performed under local anesthesia for continuous arterial blood pressure monitoring. One large-bore volume line (18-gauge) was placed. Continuous PAP, central venous monitoring, and a central venous line for vasopressors/inotropes were employed via the previously inserted PA catheter. The cardiac output monitoring was done via a FloTrac Sensor EV1000 Clinical Platform (Edwards Lifesciences Corp., USA), which provided valuable data on continuous cardiac output, cardiac index, systemic vascular resistance, and stroke volume (Table 1). As to cardiovascular support, an inotropic drug (dobutamine) and vasopressors (norepinephrine and phenylephrine) were on hand in the operating room. Vigilance for pulmonary hypertensive crisis was crucial, and iloprost and milrinone were selected as pulmonary vasodilators to deal with this event. Combined spinal-epidural anesthesia was performed at the L3–4 intervertebral space, with the patient in the lateral position. The patient received 0.5% heavy bupivacaine (4 mg; 0.8 ml), fentanyl (20 μg), and morphine (200 μg). To avoid the use of an intravascular injection, 2% xylocaine with 1:200,000 adrenaline (3 ml) was given as a test dose. The patient was then placed in the supine position with a left tilt. After performing the combined spinal-epidural anesthesia, the patient’s blood pressure was 140/80 mmHg, PAP was 60/22 mmHg, and heart rate was 84 beats/min. Anesthetic level was achieved to the 10th thoracic dermatome (T10). Before the cesarean delivery and tubal sterilization were performed, the cardiac surgeon opened the right groin to place an introducer sheath. It facilitated the insertion of a veno-arterial ECMO cannula, which would be used in the event of decompensated cardiac failure (Fig. 2). Meanwhile, epidural agents were administered incrementally over a 30-min period. It consisted of a local anesthetic agent (up to 15 ml of 2% xylocaine), 1:200,000 adrenaline, and fentanyl (30 μg). The patient then achieved an adequate anesthetic level to the 4th thoracic dermatome (T4). During surgery but before the fetal delivery, the PAP suddenly increased from 60/22 to 88/37 mmHg but with a stable systolic blood pressure. To reduce the PVR, milrinone (Primacor®, Sanofi-Synthelabo Inc, NY, USA) was initially given at 0.3 μg/kg/min, with incremental adjustments of 0.5 μg/kg/min to reduce PVR. The PAP subsequently dropped slightly to between 67–76/22–26 mmHg. The patient’s cardiac monitoring parameters are listed in Table 1.
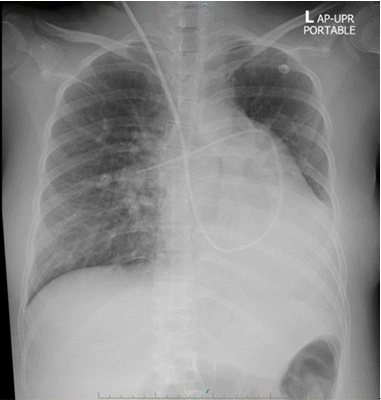
After performing anesthesia, the entire operation duration was 1 h and 5 min. The estimated blood loss was 300 ml. The central venous pressure dropped from 26 to 12 mmHg, the cardiac index decreased from 5.5 to 4.9 L/min/m2, and the systemic vascular resistance changed from 1,078 to 718 dynes/s/cm-5. This set of values indicates that the hypovolemic state occurred from acute blood loss and preoperative anemia. However, the patient’s blood pressure remained stable at around 140–170/70–90 mmHg, with her heart rate at 70–90 beats/min. One unit of packed red cells (287 ml) and 700 ml of Ringer’s lactate solution were administered. After delivery of a baby girl (birthweight, 1,540 grams; Apgar scores of 8 and 9), no oxytocin bolus was given. The patient received oxytocin (40 units) in Ringer’s lactate (1,000 ml) at a continuous intravenous infusion rate of 100 ml/h. After surgery, the patient was transferred to the respiratory intensive care unit. To manage postoperative pain, patient-controlled epidural analgesia was used, together with bupivacaine (0.05%) and morphine (0.02 mg/ml). Eight hours after surgery, diuresis was enhanced with furosemide due to increased pulmonary infiltration (Fig. 3). Sildenafil was resumed. Milrinone was discontinued on postoperative day 3. Prophylactic anticoagulation was achieved with enoxaparin after the epidural catheter was removed on postoperative day 3. The patient was discharged from the hospital on postoperative day 11.
Pregnant women with PAH who are undergoing cesarean deliveries are very high-risk cases. The anesthetic goal is to balance the systemic arterial pressure and PAP, as a lower systemic arterial pressure will compromise RV perfusion, resulting in RV failure. Hypoxia, hypercarbia, metabolic acidosis, sympathetic stimulation, and pain contribute to an increase in PAP, which leads to a PAH crisis and RV failure [8].
RV failure is characterized by an elevated RV filling pressure (> 8 mmHg) and a low cardiac index (< 2.5 L/min/m2) [6]. The probability of cardiovascular collapse during surgery is based on the preoperative determination of a poor functional class [6]. Immediately after delivery, autotransfusion leads to increased venous return, cardiac output, and PAP. These contribute to a heightening of the risk of ventricular overload, heart failure, and death in the operating room [8]. Thus, the surgery for these patients should be performed in a cardiac operating theatre. This will ensure that immediate support is available for the patient’s cardiovascular system, for example, extracorporeal membrane oxygenation, which is generally reserved for decompensated heart failure. The decision to use general anesthesia or regional anesthesia depends on a patient’s preoperative status. Both can have a detrimental impact on RV dysfunction. Anesthesiologists should therefore give careful consideration to the selection of the anesthetic technique to be employed, ensuring to balance the respective risks and benefits, and always be prepared for the possibility of precarious situations [8]. In the case of a cesarean delivery, the use of either an epidural anesthesia with slow incremental titration or a low-dosage of combined spinal-epidural anesthesia is recommended [6,9]. Regional anesthesia reduces sympathetic nerve activity and ensure adequate pain control by sensory blockage, thereby attenuating the negative effects on the cardiovascular system. However, a single-shot spinal anesthesia is contraindicated as it may result in vasodilatation, producing severe hypotension. As a result, there is a significant reduction in systemic vascular resistance [3,10]. Because it is imperative to promptly manage vasodilatation after the administration of regional anesthesia, vasopressor agents such as phenylephrine or norepinephrine should be prepared.
General anesthesia is preferred in patients who are critically ill and need airway control, and when it is deemed possible to use intraoperative transesophageal echocardiography monitoring. This is because the sudden hemodynamic change that can occur immediately after delivery can lead to rapid decompensation. Furthermore, the obvious advantages of general anesthesia are firstly, the ability to control ventilation (which avoids the hypoxia or hypercarbia that may precipitate an increase in PVR) and secondly, no effect on perioperative anticoagulant management. However, the disadvantages of endotracheal tube insertion and positive pressure ventilation are an increasing intrathoracic pressure and a rising PAP. These chiefly occur during intubation and extubation, which are when sympathetic nerve stimulation is the greatest. McMillan et al. [11] reported the case of a maternal death within 24 h of a cesarean delivery under general anesthesia in a woman with PH secondary to SLE.
Our patient had functional status III–IV with preserved RV function. We therefore opted for combined spinal-epidural anesthesia, meticulous hemodynamic monitoring, and titration of the epidural local anesthetic until an adequate anesthetic level was achieved. We were aware of the impact of spinal anesthesia alone on the cardiovascular system, as decreased systemic vascular resistance can lead to vasodilation and severe hypotension. Lin and Lu [12] reported 3 cases of pregnant patients with severe idiopathic PAH who underwent cesarean delivery and received epidural anesthesia with full monitoring. The researchers concluded that continuous epidural anesthesia is preferable to general anesthesia, and should be titrated to the 6th thoracic dermatome (T6) to provide adequate analgesia with hemodynamic stability.
The greatest concern in the operating room is a pulmonary hypertensive crisis. The mechanism involves an abrupt pulmonary vasoconstriction that causes the mPAP to exceed the mean systemic arterial pressure, resulting in RV failure and systemic hypotension [13]. Severe hypotension should be aggressively treated by optimizing the cardiac preload and the vasopressor infusion, while an inotropic drug should be administered to deal with severe heart failure associated with autotransfusion after delivery. We canulated the veno-arterial ECMO at the femoral vessels to support the cardiovascular system in the event of an RV failure. We also prepared PAH-specific vasodilators (inhaled iloprost and intravenous milrinone).
Inhaled iloprost is classified as a prostacyclin analogue. When coupled to prostanoid receptors, it causes vasodilation, decreases the mPAP and systemic vascular resistance, and suppresses platelet aggregation. Inhaled iloprost is easily administered by an ultrasonic nebulizer and is generally well tolerated. It is classified as a category C drug by the US Food and Drug Administration (animal studies have demonstrated fetal adverse effects; no human studies; and potential benefits may warrant use of the drug) [14]. As to milrinone, it is a phosphodiesterase-3 inhibitor. It increases the cyclic adenosine monophosphate levels of smooth muscle cells, thereby reducing their contractile tone. Moreover, it has positive inotropic properties which increase the cardiac index and decrease the PVR [15]. Since it is classified as category C, it is not recommended for usage with pregnant or lactating women. However, we decided to administer milrinone by intravenous infusion due to the high PAP during surgery. For this patient, we deemed that the advantages of using milrinone outweighed the possible side effects.
The cardiovascular effects of the uterotonic agents used with this patient were a major concern. We avoided using a bolus or a high concentration of oxytocin as the resulting decrease in systemic vascular resistance would result in a marked fall in blood pressure. Other uterotonic agents (such as methylergonovine and carboprost) are contraindicated as they would result in pulmonary vasoconstriction, which would be followed by an increase in the PAP and cardiovascular collapse [6].
This patient required intensive monitoring during the postoperative period. She received furosemide due to an increase in her venous return on postoperative day 1. Given that pain affects PAP, we managed her postoperative pain via a patient-controlled epidural analgesia. The choice of regional anesthesia with an epidural catheter is superior to general anesthesia for the control of pain after a cesarean delivery.
In conclusion, a pregnancy with severe PAH increases the risk of maternal morbidity and mortality. Thus, patients need to be closely monitored, and delivery should be carried out at a tertiary-care center by a multidisciplinary team. Clinicians must be fully cognizant of the physiological changes during the peripartum period, and be prepared for the possible complications. Invasive cardiovascular monitoring and scrupulous anesthetic management are essential. We recommend the intravenous infusion of milrinone perioperatively, which provides the dual benefits of lowered PVR and increased cardiac contractility.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the patient who generously agreed to depict her data in this study, Miss Chusana Rungjindamai for her assistance with documentation and study coordination.
REFERENCES
1. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019; 53:1801913.

2. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903-75. Erratum in: Eur Respir J. 2015; 46:1855–6.
4. Tselios K, Gladman DD, Urowitz MB. Systemic lupus erythematosus and pulmonary arterial hypertension: links, risks, and management strategies. Open Access Rheumatol. 2016; 9:1–9.

5. Sliwa K, van Hagen IM, Budts W, Swan L, Sinagra G, Caruana M, et al. ROPAC investigators. Pulmonary hypertension and pregnancy outcomes: data from the Registry of Pregnancy and Cardiac Disease (ROPAC) of the European Society of Cardiology. Eur J Heart Fail. 2016; 18:1119–28.

6. Svetlichnaya J, Janmohammed M, De Marco T. Special situations in pulmonary hypertension: pregnancy and right ventricular failure. Cardiol Clin. 2016; 34:473–87.
7. Weiner MM, Hamburger J, Beilin Y. The pregnant patient with cardiac disease. Kaplan's cardiac anesthesia. In : Kaplan JA, Augoustides JGT, Manecke GR, Maus T, Reich DL, editors. Philadelphia (PA): Elsevier;2016. p. 1582–99.
8. Rex S, Devroe S. Anesthesia for pregnant women with pulmonary hypertension. Curr Opin Anaesthesiol. 2016; 29:273–81.

9. Meng ML, Landau R, Viktorsdottir O, Banayan J, Grant T, Bateman B, et al. Pulmonary hypertension in pregnancy: a report of 49 cases at four tertiary North American sites. Obstet Gynecol. 2017; 129:511–20.
10. Gomar C, Errando CL. Neuroaxial anaesthesia in obstetrical patients with cardiac disease. Curr Opin Anaesthesiol. 2005; 18:507–12.

11. McMillan E, Martin WL, Waugh J, Rushton I, Lewis M, Clutton-Brock T, et al. Management of pregnancy in women with pulmonary hypertension secondary to SLE and anti-phospholipid syndrome. Lupus. 2002; 11:392–8.

12. Lin DM, Lu JK. Anesthetic management in pregnant patients with severe idiopathic pulmonary arterial hypertension. Int J Obstet Anesth. 2014; 23:289–90.

13. Brunner N, de Jesus Perez VA, Richter A, Haddad F, Denault A, Rojas V, et al. Perioperative pharmacological management of pulmonary hypertensive crisis during congenital heart surgery. Pulm Circ. 2014; 4:10–24.

14. Halpern DG, Weinberg CR, Pinnelas R, Mehta-Lee S, Economy KE, Valente AM. Use of medication for cardiovascular disease during pregnancy: JACC state-of-the-art review. J Am Coll Cardiol. 2019; 73:457–76.
15. Kirsten R, Nelson K, Kirsten D, Heintz B. Clinical pharmacokinetics of vasodilators. Part II. Clin Pharmacokinet. 1998; 35:9–36.
Fig. 2.
A cardiac surgeon inserted an introducer sheath for ECMO to the right groin after spinal-epidural anesthesia was performed; before starting cesarean delivery. ECMO: extracorporeal membrane oxygenation.
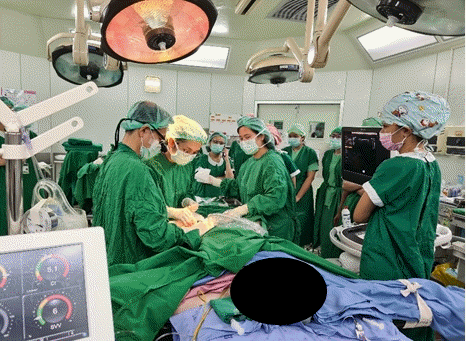
Table 1.
Patient’s Cardiac Monitoring Parameters during the Pre- and Intraoperative Periods
| Parameters | Preoperative period |
Intraoperative period* |
||||
|---|---|---|---|---|---|---|
| Initial in operating room |
After anesthesia (CSE) |
After delivery |
||||
| Time 1 | Time 2 | Time3 | Time 4 | |||
| SBP | 138 | 160 | 140 | 160 | 160 | 166 |
| DBP | 85 | 90 | 80 | 70 | 80 | 78 |
| HR | 80 | 74 | 84 | 80 | 90 | 90 |
| CVP | 13 | 22 | 22 | 26 | 11 | 12 |
| PAP | 68/17 | 66/25 | 60/22 | 88/37 | 67/22 | 76/26 |
| PCWP | 13 | 9 | NA | NA | NA | NA |
| CI | 3.68 | 5.0 | 5.5 | 5.4 | 5.1 | 4.9 |
| SVR | 1031 | 1078 | 997 | 900 | 884 | 718 |
CI: cardiac index (L/min/m2), CSE: combined spinal-epidural, CVP: central venous pressure (mmHg), DBP: diastolic blood pressure (mmHg), HR: heart rate (beats/min), PAP: pulmonary arterial pressure (mmHg), PCWP: pulmonary capillary wedge pressure (mmHg), SBP: systolic blood pressure (mmHg), SVR: systemic vascular resistance (dynes/s/cm-5), NA: not available. Time 1 = 5 min after combined spinal-epidural anesthesia administration. Time 2 = 20 min after combined spinal-epidural anesthesia administration. Time 3 = immediately after delivery. Time 4 = 15 min after delivery.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download