Abstract
Background
Reexpansion pulmonary edema is a rare but potentially lethal complication. We report a case of suspected reexpansion pulmonary edema that led to cardiac arrest.
Case
A 16-year-old male patient underwent wedge resection due to right pneumothorax. The patient showed pink frothy sputum three hours following surgery, and a chest x-ray showed right unilateral pulmonary edema. Thirteen hours following surgery, the patient continuously showed pink frothy sputum and presented with severe hypoxemia, tachypnea, and tachycardia. After transferring to the intensive care unit (ICU), he developed ventricular tachycardia. Cardiopulmonary resuscitation was performed for 32 min. Chest X-ray showed diffuse bilateral pulmonary edema. Extracorporeal membrane oxygenation was performed. During the 65 days of ICU care, the patient became mentally alert. However, follow-up echocardiography revealed severe heart failure.
Patients may develop pulmonary edema following thoracostomy tube insertion and elective wedge resection for spontaneous pneumothorax that ultimately progresses to diffuse bilateral pulmonary edema and cardiac arrest. Among various differential diagnoses, reexpansion pulmonary edema is the most suspected diagnosis. Reexpansion pulmonary edema is a rare but fatal complication of thoracostomy tube insertion. It can also occur after one-lung ventilation [1]. Clinically, it can be suspected when the patient shows pink, frothy sputum with tachypnea, tachycardia, and hypoxia. In general, patients show unilateral pulmonary edema on the ipsilateral side, and supportive care is provided for management. However, severe cases can progress to bilateral pulmonary edema, which requires endotracheal intubation and mechanical ventilation.
However, our patient required extracorporeal membrane oxygenation (ECMO) because supportive care was ineffective, and the patient was successfully resuscitated. Thus, we report the case with a relevant literature review to contribute to managing severe reexpansion pulmonary edema. Our Institutional Review Board (no. EMC 2021-05-007) approved this case report and waived the requirement for written informed consent.
A 16-year-old male patient (height 179 cm, weight 60.8 kg) was admitted to the thoracic surgery department with a complaint of sudden chest pain that occurred two days ago. The patient had no medical history and was a never-smoker. The patient was diagnosed with 50% right pneumothorax (Fig. 1); hence, a 24 French thoracostomy tube was inserted along with the application of 20 cmH2O negative pressure. The patient was subsequently admitted for elective thoracoscopic wedge resection. Laboratory test and electrocardiogram (ECG) findings did not reveal any abnormalities, and the patient’s American Society of Anesthesiologists physical status classification was 1.
Vital signs measured immediately after arriving at the operating room were as follows: blood pressure (BP), 148/85 mmHg; heart rate (HR), 85 beats/min; SpO2, 100%; and temperature, 36.6°C. Total intravenous anesthesia was induced using propofol and remifentanil for induction and maintenance. After the loss of consciousness, succinylcholine 100 mg was administered, and endotracheal intubation was performed using a 37 French left-sided double-lumen tube (HumanBroncho®, Insung Medical, Korea) using a laryngoscope. While maintaining the fraction of inspired oxygen (FiO2) at 0.5, mechanical ventilation was performed to maintain the end-tidal carbon dioxide (EtCO2) at 30–33 mmHg. The peak airway pressure was maintained under 25 cmH2O. Vecuronium (4 mg) was administered to maintain a neuromuscular blockade. During surgery, multiple bullae were discovered in the apex and the posterior segments of the right upper lobe. Therefore, wedge resection was performed. When the surgical procedure was completed, a 10-s recruitment maneuver was performed twice with a peak airway pressure of 30 cmH2O to reexpand the collapsed lung; the surgery lasted 55 min and was completed without any complications. The total fluid infusion volume was 400 ml, including 300 ml of hydroxyethyl starch (Volulyte®, Fresenius Kabi Deutschland GmbH, Germany) and 100 ml of Hartmann solution.
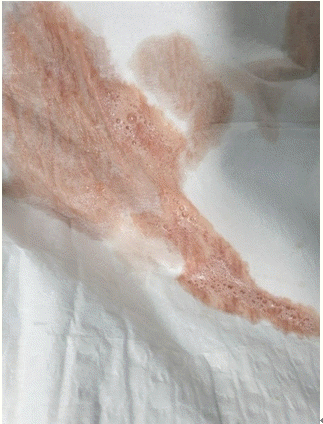
Immediately after the arrival at the post-anesthesia care unit, the patient’s vital signs were as follows: BP, 161/112 mmHg; HR, 109 beats/min; SpO2, 95%; temperature, 36.5℃; and respiration rate, 25 breaths/min. The patient complained of nausea immediately after arrival at the post-anesthesia care unit, which did not resolve after administration of 0.075 mg of palonosetron hydrochloride (Aloxi®, Helsinn Birex Pharmaceuticals Ltd., Ireland), and 5 mg of dexamethasone. Approximately 10 min later, the patient vomited pink frothy sputum several times (Fig. 2). After observing the patient for 60 min, the patient showed improvement in nausea, and the vital signs with O2 5 L/min using an oxygen reserve bag mask were as follows: BP, 145/88 mmHg; HR, 98 beats/min; SpO2, 99%; temperature, 36.5℃; and respiration rate, 19 breaths/min. The patient’s Postanesthetic Alderte recovery score was 9; hence, he was sent to the ward with continued oxygen supplementation.
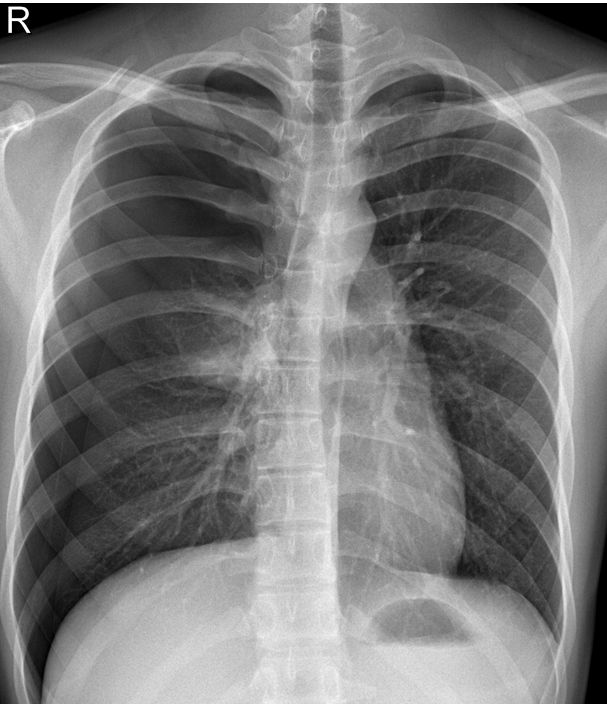
Chest radiography performed 3 h following surgery showed right pulmonary edema (Fig. 3A), and the vital signs were: BP, 154/90 mmHg; HR, 115 beats/min; SpO2, 95%; and temperature, 37.5℃. The patient was alert; however, he showed continuous agitation. In addition, a postoperative laboratory test revealed that the hemoglobin concentration had increased to 19.9 g/dl compared to 17.3 g/dl before the surgery. Thirteen hours following surgery, the patient continuously began to cough pink frothy sputum, developed dyspnea, and presented with mental disorientation. His vital signs were as follows: BP, 185/129 mmHg; HR, 191 beats/min; SpO2, 78%; and temperature, 39.2℃. The patient’s extremities were pale, and although arterial blood gas analysis was attempted, the radial artery pulse could not be detected. Blood vessel puncture was successful; however, only a trace amount of darkened blood was aspirated; hence, we thought that venous blood was sampled, and the test was not performed. Endotracheal intubation was performed immediately, and the patient was transferred to the intensive care unit. Thirty minutes later, SpO2 dropped to 45% at FiO2 of 0.6, with ECG showing ventricular tachycardia; therefore, cardiopulmonary resuscitation (CPR) was performed. While performing chest compressions, 1 mg of epinephrine was injected every three minutes (total 10 mg), and recovery of spontaneous circulation (ROSC) was achieved after five consecutive direct-current cardioversion at 200 J. CPR was performed for 32 min. Chest radiography performed after achieving ROSC showed diffuse bilateral pulmonary edema (Fig. 3B), and echocardiography revealed moderate left ventricular systolic dysfunction with global hypokinesia and a left ventricle ejection fraction (LVEF) of 35%. Vital signs were BP, 61/48 mmHg; and HR, 152 beats/min. Thus, veno-veno-arterial ECMO was performed via a femoral approach. For inotropic support, dopamine (20 μg/kg/min), dobutamine (20 μg/kg/min), and epinephrine (0.4 μg/kg/min) were used in addition to norepinephrine (1.6 μg/kg/min) as a vasopressor. In addition, there was no urine output even after 2 h of inserting a 14 French Foley catheter; thus, continuous renal replacement therapy (CRRT) was initiated, and continuous veno-venous hemodiafiltration mode was used to enable simultaneous hemodialysis and hemofiltration.
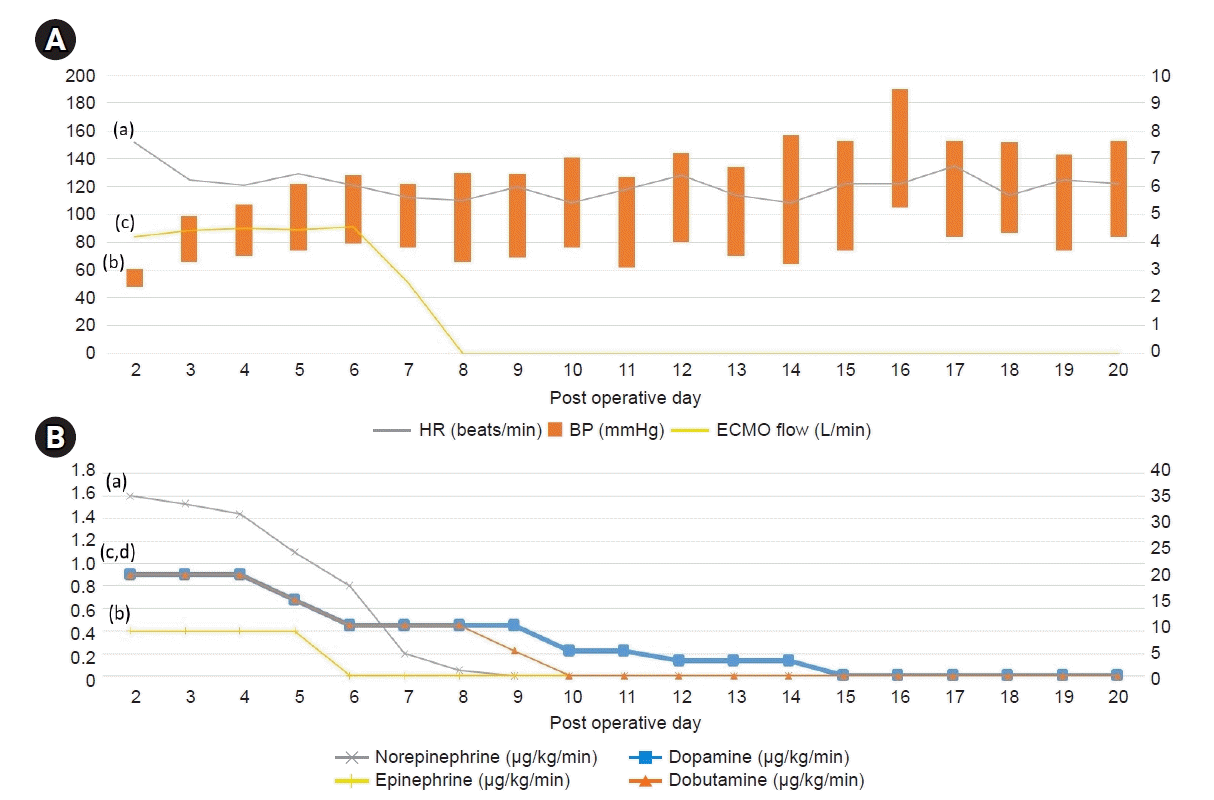
From post-operative day (POD) 3 to POD 11, the infusion drugs were tapered with continuous supportive care and blood transfusion (Fig. 4). ECMO was maintained at 4.4–4.6 L/min and terminated on POD 9. On POD 11, the patient’s mental status improved from stupor to drowsy, and he regained orientation; motor grade improved to II, with the patient being able to obey commands. On POD 15, all the infusion drugs were terminated, and the vital signs were BP, 130/62 mmHg; HR, 114 beats/min; SpO2, 100%; and temperature, 37.5℃ (Fig. 4).
On POD 27, follow-up echocardiography revealed severe left ventricular dysfunction with global hypokinesia, with a further decrease in LVEF to 22%. On POD 44, urine output recovered to 1,645 ml/day, based on which CRRT was weaned. His vital signs were stable. On POD 50, the patient became alert, and extubation was performed on POD 55. On POD 66, follow-up echocardiography revealed an LVEF of 20%.
When a patient shows clinical signs and chest radiography of pulmonary edema, clinicians must differentiate the cause. We suspected the following: Excessive fluid replacement, negative pressure pulmonary edema, pulmonary thromboembolism, reexpansion pulmonary edema.
There is a possibility that 400 ml of fluid over 55 min of surgery may have caused pulmonary edema because a routine fluid replacement for nil-per os time is not required [2]. Negative pulmonary edema may also have occurred by upper airway obstruction. However, during extubation, we did not observe the patient biting the endotracheal tube or increased peak airway pressure [3]. Also, pulmonary ischemia caused by pulmonary thromboembolism could not be ruled out; however, the patient did not complain of chest pain [4].
We assumed that the reexpansion pulmonary edema was the most probable diagnosis. The patient had not only several risk factors of reexpansion pulmonary edema (16 years of age, more than 50% of pneumothorax, 20 cmH2O of negative pressure, one-lung ventilation), but also typical symptoms of reexpansion pulmonary edema.
Reexpansion pulmonary edema is a rare condition with an incidence of < 1%. It usually occurs in patients with pneumothorax, pleural effusion, or severe atelectasis following the insertion of a thoracostomy tube [5]. Also, similar to this case, there are several reports of reexpansion pulmonary edema occurring after one-lung ventilation during thoracic surgery [1,6]. More than half of the patients develop symptoms within an hour, while others develop symptoms within 24 h [5]. Patients present with tachypnea, tachycardia, and hypoxia, and do not respond to oxygen therapy owing to the shunt caused by fluid-filled alveoli. A large third space in the lungs may also cause hypotension [7]. Most patients show progressive unilateral pulmonary edema, and bilateral contralateral edema is uncommon. However, without proper treatment, the contralateral lung may be involved. Risk factors for reexpansion pulmonary edema include persistent pneumothorax for 7 days or longer, younger age (aged 20–39 years), pneumothorax > 30% of the lung field, and method of reexpansion [8].
The pathophysiology of reexpansion pulmonary edema remains unknown; however, it is believed that multiple factors act on the capillary bed and increase its permeability [9]. A recent study reported that inflammatory mediators, such as interleukin-8 and monocyte chemoattractant protein-1, are involved in pathogenesis [10].
Management of reexpansion pulmonary edema is generally supportive. Prompt recognition is important, and oxygen supplementation can help treat mild symptoms. In addition, most cases show unilateral pulmonary edema; hence, the lateral decubitus position with the affected side up could be beneficial [11]. There was also a report of symptomatic improvement in patients with severe reexpansion pulmonary edema using methylprednisolone pulse therapy (1,000 mg/day for 3 days) [12].
In the present case, the patient showed typical clinical features of reexpansion pulmonary edema after being transferred to the post-anesthesia care unit and was thus placed under observation for an hour. He did not show any further exacerbation of symptoms; hence, he was sent to the ward with continued oxygen supplementation. A chest radiograph taken 2 h after returning to the ward only showed right unilateral pulmonary edema; however, considering the worsening of tachycardia, tachypnea, and hypoxemia over a few hours, rapid contralateral lung involvement should have been considered.
The authors thought that his low alveolar PO2 caused by fluid-filled alveoli might trigger hypoxic pulmonary vasoconstriction (HPV). HPV can aggravate pulmonary hypertension, resulting in rupture of the basement membrane [13]. Furthermore, younger patients have higher cardiac autonomic function, represented by heart rate variability [14]. This can cause extravasation of fluid from capillaries and worsen pulmonary edema. The patient’s hemoglobin concentration increased after surgery compared to the preoperative level (17.3 → 19.9 g/dl), confirming the extravasation. Judging from these signs, we can assume the high possibility of worsened reexpansion pulmonary edema, especially in younger patients (< 40 years). Therefore, even with mild reexpansion pulmonary edema, close monitoring and oxygen supplementation are crucial.
Regarding his LVEF of 35% on POD 2, because the patient had a metabolic equivalent of task score of 6 or higher before the surgery, it is difficult to conclude that there was an unknown cardiopulmonary disease. According to one study, a higher resting HR and temporal increase in HR increase the risk of adverse outcomes [15]. Therefore, there is a possibility that the patient’s persistent tachycardia had caused stress cardiomyopathy. Furthermore, CPR itself may have caused irreversible myocardial damage.
The limitation of this case report is that although reexpansion pulmonary edema was suspected early on, it could have been a better if there had been further evaluation such as chest computed tomography or pulmonary perfusion scan to rule out pulmonary thromboembolism. Furthermore, we cannot confirm whether a respiratory failure led to cardiac arrest because the patient did not receive preoperative cardiac evaluation due to his young age and no underlying diseases.
In conclusion, we discovered that reexpansion pulmonary edema could quickly progress to diffuse bilateral pulmonary edema even with mild symptoms. Thus, even patients with mild reexpansion pulmonary edema must be closely monitored to ensure immediate treatment, and if necessary, appropriate treatment to lower autonomic function such as sedation should be provided. Furthermore, it should be noted that prompt ECMO could be an option for improving the patient’s survival.
Notes
REFERENCES
1. Sugiyama Y, Shimizu F, Shimizu S, Urasawa M, Tanaka S, Kawamata M. Severe re-expansion pulmonary edema induced by one-lung ventilation. Respir Care. 2015; 60:e134–40.

2. Field RR, Mai T, Hanna S, Harrington B, Calderon MD, Rinehart J. Lack of impact of nil-per-os (NPO) time on goal-directed fluid delivery in first case versus afternoon case starts: a retrospective cohort study. BMC Anesthesiol. 2019; 19:191.

3. Bhattacharya M, Kallet RH, Ware LB, Matthay MA. Negative-pressure pulmonary edema. Chest. 2016; 150:927–33.

4. Righini M, Robert-Ebadi H. Diagnosis of acute pulmonary embolism. Hamostaseologie. 2018; 38:11–21.

5. Sherman SC. Reexpansion pulmonary edema: a case report and review of the current literature. J Emerg Med. 2003; 24:23–7.

6. Matsumiya N, Dohi S, Kimura T, Naito H. Reexpansion pulmonary edema after mediastinal tumor removal. Anesth Analg. 1991; 73:646–8.

7. Cinnella G, Dambrosio M, Brienza N, Ranieri VM. Reexpansion pulmonary edema with acute hypovolemia. Intensive Care Med. 1998; 24:1117.

8. Matsuura Y, Nomimura T, Murakami H, Matsushima T, Kakehashi M, Kajihara H. Clinical analysis of reexpansion pulmonary edema. Chest. 1991; 100:1562–6.

9. Trachiotis GD, Vricella LA, Aaron BL, Hix WR. As originally published in 1988: Reexpansion pulmonary edema. Updated in 1997. Ann Thorac Surg. 1997; 63:1206–7.
10. Sakao Y, Kajikawa O, Martin TR, Nakahara Y, Hadden WA 3rd, Harmon CL, et al. Association of IL-8 and MCP-1 with the development of reexpansion pulmonary edema in rabbits. Ann Thorac Surg. 2001; 71:1825–32.

11. Kasmani R, Irani F, Okoli K, Mahajan V. Re-expansion pulmonary edema following thoracentesis. CMAJ. 2010; 182:2000–2.

12. Yoshikawa K, Miyata M, Sueoka N, Yamamoto D. Effective steroid therapy for reexpansion pulmonary edema. JMA J. 2019; 2:97–8.

13. Dunham-Snary KJ, Wu D, Sykes EA, Thakrar A, Parlow LRG, Mewburn JD, et al. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest. 2017; 151:181–92.
Fig. 3.
(A) Chest x ray taken three hours following surgery showing right side limited unilateral pulmonary edema. (B) Thirteen hours following surgery showing diffuse bilateral pulmonary edema.

Fig. 4.
Vital signs, extracorporeal membrane oxygenation (ECMO) flow, inotropic agent, vasopressor trends during ECMO institution. (A-a) Heart rate (HR) (beats/min), (A-b) blood pressure (BP) (mm/Hg), (A-c) ECMO flow (L/min), (B-a) norepinephrine (μg/kg/min), (B-b) epinephrine (μg/kg/min), (B-c) dopamine (μg/kg/min), (B-d) dobutamine (μg/kg/min). Note that on postoperative day #9, not only epinephrine, and norepinephrine, but also ECMO was terminated. ECMO was maintained for 7 days. After that, dopamine and dobutamine were tapered while maintaining stable vital signs.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download