Abstract
Transient splenial lesion of the corpus callosum can be observed in various diseases such as cancer, drug use, metabolic disorders, and cerebrovascular disorders, as well as in patients with infectious diseases. During the coronavirus disease 2019 (COVID-19) pandemic, there were increasing reports of these lesions being detected on brain imaging tests performed in patients with neurological symptoms. On brain magnetic resonance imaging, findings suggestive of cytotoxic edema are observed in the splenium; these are known to disappear with improvement of clinical symptoms. Cytokinopathy caused by infection increases the permeability of the blood–brain barrier and activates the glial cells of the brain to induce cytotoxic edema. Most patients have a good prognosis. The causes, mechanism, diagnosis, treatment and prognosis of transient splenial lesions of the corpus callosum will be summarized in this review.
The corpus callosum is a thick bundle of nerve fibers connecting both the cerebral hemispheres. The splenium is located in the posterior part of the corpus callosum and contains crossing axonal fibers from the occipito-parietal and temporal cortex [1,2]. Transient lesions of the splenium are reported in a variety of cytotoxic lesions of the corpus callosum (CLOCC), including mild encephalitis/encephalopathy with a reversible isolated SCC lesion (Middle East respiratory syndrome [MERS]), and reversible splenial lesion syndrome (RESLES) [3-5]. Lesions in the splenium of the corpus callosum are associated with various diseases, including infection, metabolic disturbance, drug use, epilepsy, malignancies, cerebrovascular disease, and trauma [6-10]. Recently, various neurological complications related to coronavirus disease 2019 (COVID-19) have been reported, and among them, there was a case in which a transient splenial lesion was observed [11]. In this article, we review the transient splenial lesions observed in various infectious diseases such as COVID-19.
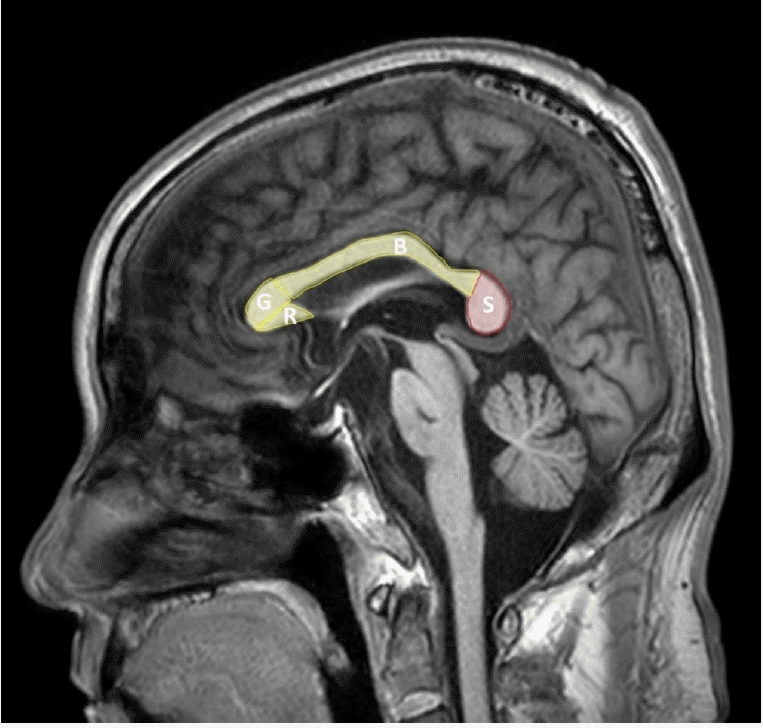
The corpus callosum is a fiber connecting the left and right cerebral hemispheres and is composed of four parts: the rostrum, genu, body, and splenium (Figure 1). Among them, the splenium contains fibers connecting the temporal and posterior parietals and the temporal cortex, during the 8th and 20th weeks of gestation period, the corpus callosum development is formed by development of the callosal precursors and complete by the age of four [12]. The internal carotid artery provides an arterial supply to most of the corpus callosum, but splenium receives blood suppls by the anterior pericallosal artery of the anterior circulation and the posterior pericallosal artery and posterior accessory pericallosal artery of the posterior circulation [1]. Splenium contains fibers connecting the temporal, parietal, and occipital cortex in both cerebral hemispheres, and thus is responsible for related functions. Callosotomy has been performed since 1940 for the treatment of epilepsy, and its function has been elucidated since then, and it is known that it is mainly related to visuospatial information transfer, language, reading and calculation, IQ, behavior and consciousness [13].
Transient splenial lesions are observed in various diseases and conditions and can be classified as follows: infectious disease, drug and toxic substance-related, metabolic disturbance, functional brain disease, malignancy, vascular disease, trauma, and miscellaneous (Table 1). According to a report published in 2011, the most common associated condition was epilepsy, followed by infection [5]. Among the recently reported cases, there are many reports related to infection, which include not only viral and bacterial infections, but also various infectious diseases such as mycoplasma, malaria, and dengue fever [14-17]. Infection-related cases are increasing, especially after the outbreak of COVID-19 [18-20]. According to a recent study, brain imaging was performed on 167 patients with neurological symptoms among 3,403 COVID-19 patients, and it was reported that splenium lesions were the most common among them [21]. The incidence of reversible splenium lesions is not precisely known, but it has been reported in several studies. In a study with 450 subjects, the prevalence was up to 3%, and in another study with 5,078 su, 30 splenial lesions were observed [22,23]. However, as magnetic resonance imaging (MRI) is difficult to perform in all patients, this result may be underestimated.
The clinical symptoms of patients with transient splenial lesions are nonspecific and depend on the underlying disease. Many patients show symptoms that may suggest encephalopathy or encephalitis. The most common symptom is fever, which often appears as a prodromal symptom before the onset of neurological symptoms, and symptoms such as headache, vomiting, and diarrhea are also common [24]. In addition, altered mental status, seizures, confusion, behavioral change, acute urinary retention, and delirium are known common neurological symptoms. Motor deterioration, slurred speech, neck stiffness, coma, tremor, ataxia, somnolence, dysarthria, visual disturbance, and dizziness have also been reported [24-26]. However, there are cases where only a headache or fever is present without neurological symptoms [27]. Clinical symptoms of transient splenial lesions usually fully disappear within one month. Only isolated reversible lesions have a good prognosis [26]; however, patients with severe neurologic manifestations have a poor prognosis regardless of lesion improvement [27].
Typical MRI features are reversible hyperintense signal change on T2 weighted images, fluid-attenuated inversion recovery images, diffusion-weighted images, decreased apparent diffusion coefficient (ADC) values on ADC map, and hyper-isointense signals on T1-weighted images without contrast enhancement (Figure 2) [26]. Most imaging findings disappear within 2 weeks [26]. These type of MRI findings suggest cytotoxic edema, and some studies have reported that they leave neurological sequelae, but most of them disappear completely without sequelae [4]. According to the lesion type, size, and location, they are classified into two patterns as follows: (1) a small round or oval lesion, isolated in the center of splenium and (2) a lesion in the splenium expanding into the adjacent cerebral white matter or a lesion in the splenium extending into the anterior portion of the corpus callosum (the boomerang sign) [4].
The exact pathophysiological mechanism is not well understood. Hypotheses include intramyelinic edema, inflammatory infiltrates, hyponatremia, oxidative stress, neuroaxonal damage, autoimmune process, and cytotoxic edema [4,5,26,28-30]. When cytotoxic edema is described as a mechanism, it is sometimes referred to as “cytotoxic lesions of the corpus callosum” (or “CLOCC”) based on this [4]. When inflammatory cytokines are released they can cause overexpression of the excitatory neurotransmitter glutamate, which ultimately leads to cellular swelling and cytotoxic edema due to trauma, inflammation, infection, metabolic derangement, and other associated conditions [4]. Compared with other parts of the brain, the neurons, astrocytes, and oligodendrocytes of the corpus callosum and splenium have a higher density of cytokine receptors, glutamate, and other excitatory amino acid receptors, toxin receptors, and drug receptors. Therefore, the corpus callosum and splenium may be prone to cytotoxic edema [31].
Reported treatment for transient splenial lesions vary. There have been reports of immunotherapy, such as steroids and immunoglobulin, along with supportive care for the underlying disease, or treatment with prophylactic antibiotics and antivirals [24,28]. However, no differences were observed in clinical recovery and prognosis depending on the treatment method [28].
Transient splenial lesions have been identified alongside various infections including viral (influenza, rotavirus, measles, adenovirus, human parvovirus B19, cytomegalovirus, varicella‐zoster, adenovirus, rubella, human herpesvirus‐6, human herpesvirus‐7, human immunodeficiency virus, mumps, parainfluenza, enterovirus, Epstein–Barr virus, Hantaan virus), bacterial (Legionella pneumophila, Streptococcus pneumoniae, Salmonella enteritidis, Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae, Campylobacter jejuni), and others (Mycoplasma pneumoniae, malaria, dengue fever). Although the incidence rate in patients with infectious diseases is unknown, it is reportedly low. One study reported transient splenial lesions in 13 (1.1%) out of 1,177 children with encephalitis in a large prospective data study [24,26]. In a study of COVID-19 patients, splenial lesions were observed in three out of 73 (4.1%) COVID-19 patients who underwent MRI [31]. According to studies confirming the occurrence of brain lesions during the COVID-19 pandemic using brain imaging, macrohemorrhage or microvascular injury of subcortical white and deep white matter were observed as along with decreased diffusion of the corpus callosum [21,32,33]. Although most studies have been conducted on severely ill patients or patients with neurological symptoms, considering that there are cases found even in patients without neurological symptoms, the actual incidence of brain lesions is thought to be higher, and the incidence of transient splenial lesions are also expected to be higher. Development of viral or bacterial infectious diseases is known to induce cytotoxic edema by increasing the permeability of the blood–brain barrier and activating glial cells after infection, similar to the mechanism underlying cytotoxic edema resulting from causes other than infection [31]. However, in the case of COVID-19 patients, it was also reported that the ischemic nature may be caused by hypercoagulability [34].
Transient splenial lesion of the corpus callosum was previously recognized as an imaging finding of encephalitis or encephalopathy, but recently it has been reported that it can occur in various clinical situations. With the recent COVID-19 pandemic, reports of associations with viral diseases are increasing. The prognosis is good in most cases, and brain imaging can be helpful for identifying transient splenial lesions in patients who present with an infectious disease accompanied by neurological abnormalities, and can also help determine treatment and predict the prognosis of patients by differentiating stroke, etc.
▪ Transient splenial lesion of the corpus callosum is associated with various diseases including infections.
▪ Transient splenial lesions of the corpus callosum can be diagnosed using brain magnetic resonance imaging examination.
▪ Transient splenial lesions of the corpus callosum have also been observed in some patients with coronavirus disease 2019 (COVID-19).
Notes
REFERENCES
1. Knyazeva MG. Splenium of corpus callosum: patterns of interhemispheric interaction in children and adults. Neural Plast. 2013; 2013:639430.
2. Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology. 2010; 52:447–77.

3. Takanashi J, Hirasawa K, Tada H. Reversible restricted diffusion of entire corpus callosum. J Neurol Sci. 2006; 247:101–4.

4. Starkey J, Kobayashi N, Numaguchi Y, Moritani T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. Radiographics. 2017; 37:562–76.

5. Garcia-Monco JC, Cortina IE, Ferreira E, Martínez A, Ruiz L, Cabrera A, et al. Reversible splenial lesion syndrome (RESLES): what's in a name? J Neuroimaging. 2011; 21:e1–14.

6. Takanashi J, Barkovich AJ, Shiihara T, Tada H, Kawatani M, Tsukahara H, et al. Widening spectrum of a reversible splenial lesion with transiently reduced diffusion. AJNR Am J Neuroradiol. 2006; 27:836–8.
7. Bulakbasi N, Kocaoglu M, Tayfun C, Ucoz T. Transient splenial lesion of the corpus callosum in clinically mild influenza-associated encephalitis/encephalopathy. AJNR Am J Neuroradiol. 2006; 27:1983–6.
8. Singh P, Gogoi D, Vyas S, Khandelwal N. Transient splenial lesion: further experience with two cases. Indian J Radiol Imaging. 2010; 20:254–7.

9. Malhotra HS, Garg RK, Vidhate MR, Sharma PK. Boomerang sign: clinical significance of transient lesion in splenium of corpus callosum. Ann Indian Acad Neurol. 2012; 15:151–7.

10. Cho JS, Ha SW, Han YS, Park SE, Hong KM, Han JH, et al. Mild encephalopathy with reversible lesion in the splenium of the corpus callosum and bilateral frontal white matter. J Clin Neurol. 2007; 3:53–6.

11. Kakadia B, Ahmed J, Siegal T, Jovin TG, Thon JM. Mild encephalopathy with reversible splenium lesion (MERS) in a patient with COVID-19. J Clin Neurosci. 2020; 79:272–4.

12. Kahilogullari G, Comert A, Ozdemir M, Brohi RA, Ozgural O, Esmer AF, et al. Arterial vascularization patterns of the splenium: an anatomical study. Clin Anat. 2013; 26:675–81.

13. Mathews MS, Linskey ME, Binder DK, William P. Van Wagenen and the first corpus callosotomies for epilepsy. J Neurosurg. 2008; 108:608–13.
14. Lin D, Rheinboldt M. Reversible splenial lesions presenting in conjunction with febrile illness: a case series and literature review. Emerg Radiol. 2017; 24:599–604.

15. Talukder NT, Feezel A, Lankford JE. Mild encephalitis/encephalopathy with a reversible splenial lesion associated with systemic Mycoplasma pneumoniae infection in North America: a case report. J Med Case Rep. 2022; 16:74.

16. Mawatari M, Kobayashi T, Yamamoto S, Takeshita N, Hayakawa K, Kutsuna S, et al. Mild encephalitis/encephalopathy with a reversible splenial lesion due to Plasmodium falciparum malaria: a case report. Trop Med Health. 2018; 46:37.

17. Sathananthasarma P, Weeratunga PN, Chang T. Reversible splenial lesion syndrome associated with dengue fever: a case report. BMC Res Notes. 2018; 11:412.

18. Varol F, Ergul N, Sahin EG, Can YY, Ergul U, Guven S, et al. Can plasma exchange therapy be an option for the treatment of SARS-CoV-2 related splenial lesion syndrome: two cases from the pediatric intensive care unit. Transfus Apher Sci. 2022; 103491.

19. Arıkan FA, Akdağ G, Çetiner M, Uysal N, Kabay SC. Isolated corpus callosum lesion associated with cytokine storm in COVID-19. Proc (Bayl Univ Med Cent). 2022; 35:337–8.

20. DE Oliveira FA, DE Melo TF, Rocha-Filho PA. Transient lesion in the splenium of the corpus callosum associated with COVID-19. Arq Neuropsiquiatr. 2020; 78:738.

21. Sawlani V, Scotton S, Nader K, Jen JP, Patel M, Gokani K, et al. COVID-19-related intracranial imaging findings: a large single-centre experience. Clin Radiol. 2021; 76:108–16.

22. McLeod NA, Williams JP, Machen B, Lum GB. Normal and abnormal morphology of the corpus callosum. Neurology. 1987; 37:1240–2.

23. Park MK, Hwang SH, Jung S, Hong SS, Kwon SB. Lesions in the splenium of the corpus callosum: clinical and radiological implications. Neurol Asia. 2014; 19:79–88.
24. Chen WX, Liu HS, Yang SD, Zeng SH, Gao YY, Du ZH, et al. Reversible splenial lesion syndrome in children: retrospective study and summary of case series. Brain Dev. 2016; 38:915–27.

25. Baek SH, Shin DI, Lee HS, Lee SH, Kim HY, Shin KS, et al. Reversible splenium lesion of the corpus callosum in hemorrhagic fever with renal failure syndrome. J Korean Med Sci. 2010; 25:1244–6.

26. Tada H, Takanashi J, Barkovich AJ, Oba H, Maeda M, Tsukahara H, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004; 63:1854–8.

27. Doherty MJ, Jayadev S, Watson NF, Konchada RS, Hallam DK. Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol. 2005; 62:433–7.

28. Takanashi J, Tada H, Maeda M, Suzuki M, Terada H, Barkovich AJ. Encephalopathy with a reversible splenial lesion is associated with hyponatremia. Brain Dev. 2009; 31:217–20.

29. Miyata R, Tanuma N, Hayashi M, Imamura T, Takanashi J, Nagata R, et al. Oxidative stress in patients with clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS). Brain Dev. 2012; 34:124–7.

30. Motobayashi M, Fukuyama T, Okuno-Yuguchi J, Tsukahara K, Nagaharu S, Hagimoto R, et al. Subclinical neuroaxonal damage in patients with clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Pediatr Neurol. 2017; 74:e3–4.

31. Moritani T, Smoker WR, Sato Y, Numaguchi Y, Westesson PL. Diffusion-weighted imaging of acute excitotoxic brain injury. AJNR Am J Neuroradiol. 2005; 26:216–28.
32. Conklin J, Frosch MP, Mukerji SS, Rapalino O, Maher MD, Schaefer PW, et al. Susceptibility-weighted imaging reveals cerebral microvascular injury in severe COVID-19. J Neurol Sci. 2021; 421:117308.

33. Sparr SA, Bieri PL. Infarction of the splenium of the corpus callosum in the age of COVID-19: a snapshot in time. Stroke. 2020; 51:e223–6.
34. Chougar L, Shor N, Weiss N, Galanaud D, Leclercq D, Mathon B, et al. Retrospective observational study of brain MRI findings in patients with acute SARS-CoV-2 infection and neurologic manifestations. Radiology. 2020; 297:E313–23.
Figure 2.
Two types of transient splenial lesions of the corpus callosum. Splenial lesions produce a high signal intensity on T2 diffusion-weighted imaging, a low signal intensity on T1 imaging, and decreased apparent diffusion on a coefficient map. (A) Small round lesion in the center of the splenium (arrows). (B) Boomerang sign. Lesion in the splenium extending into the adjacent cerebral white matter (arrowheads).
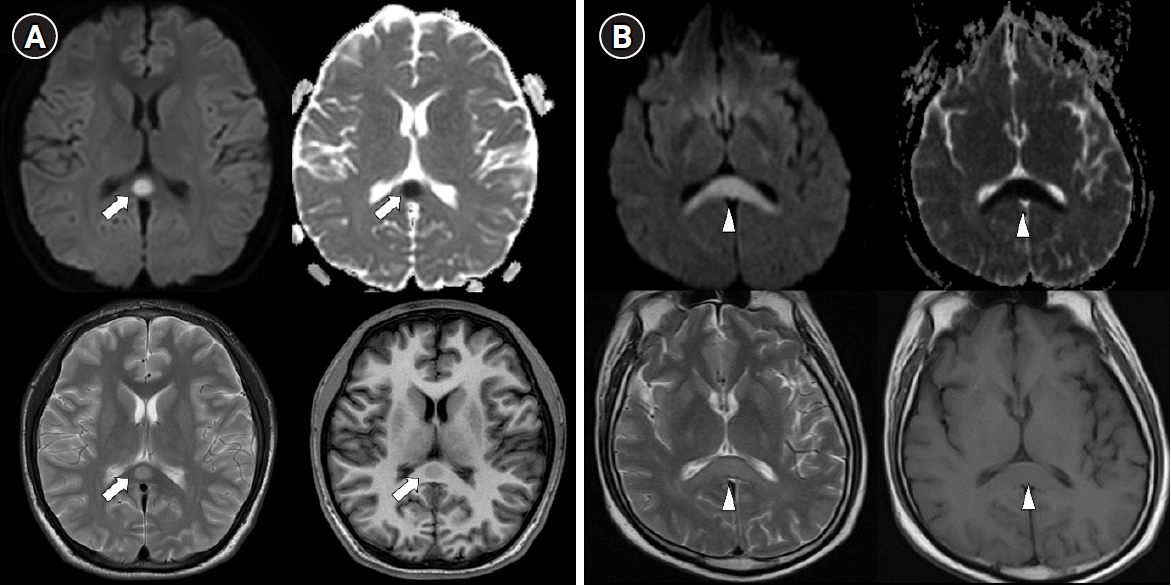
Table 1.
Causative etiology of transient splenial lesion of the corpus callosum




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download