Abstract
Background
Methods
Results
REFERENCES
Figure 1.
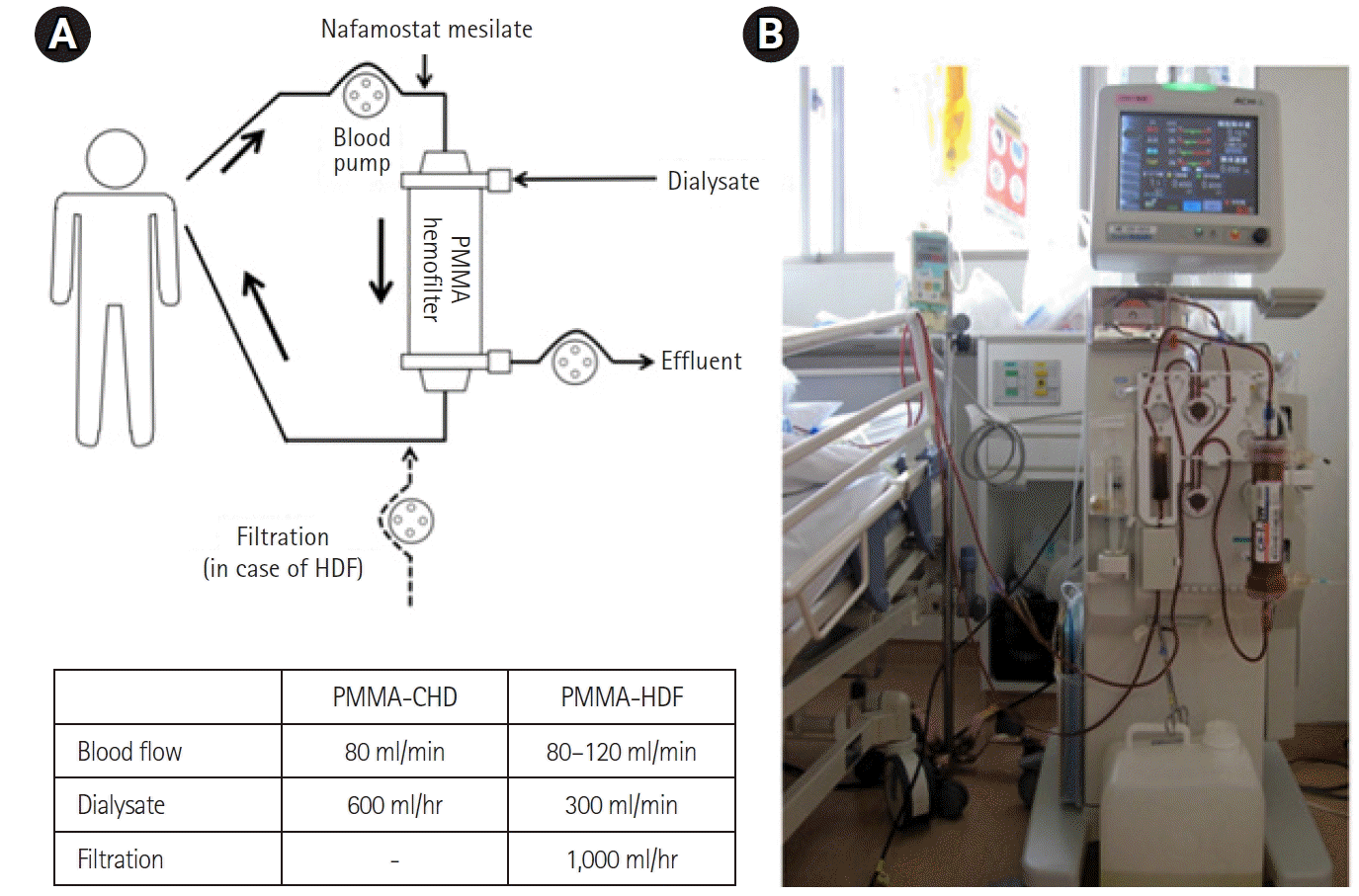
Figure 2.
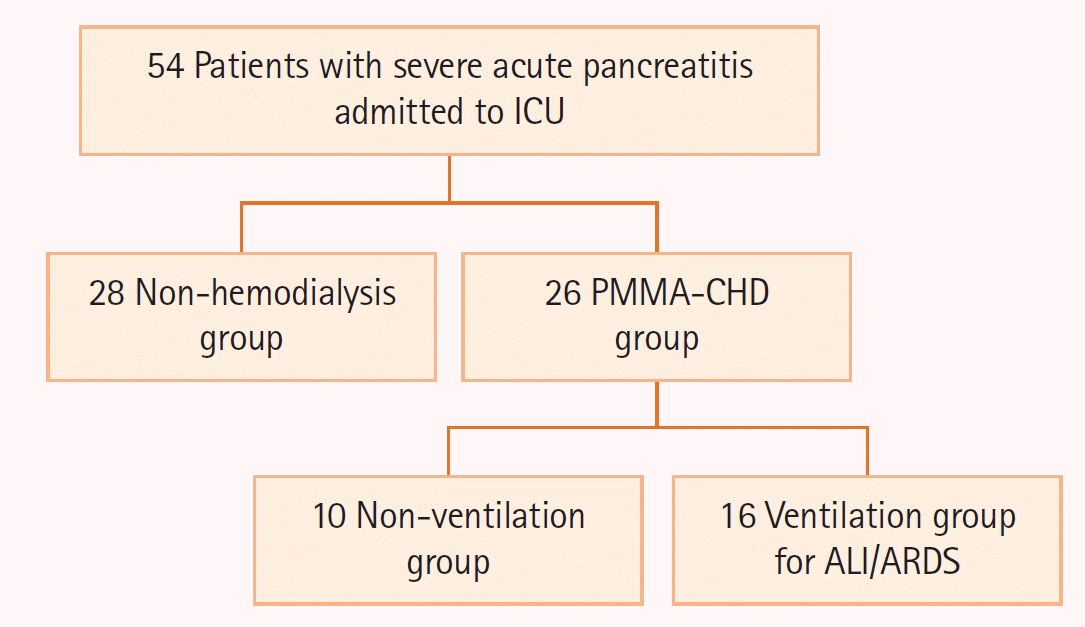
Figure 3.
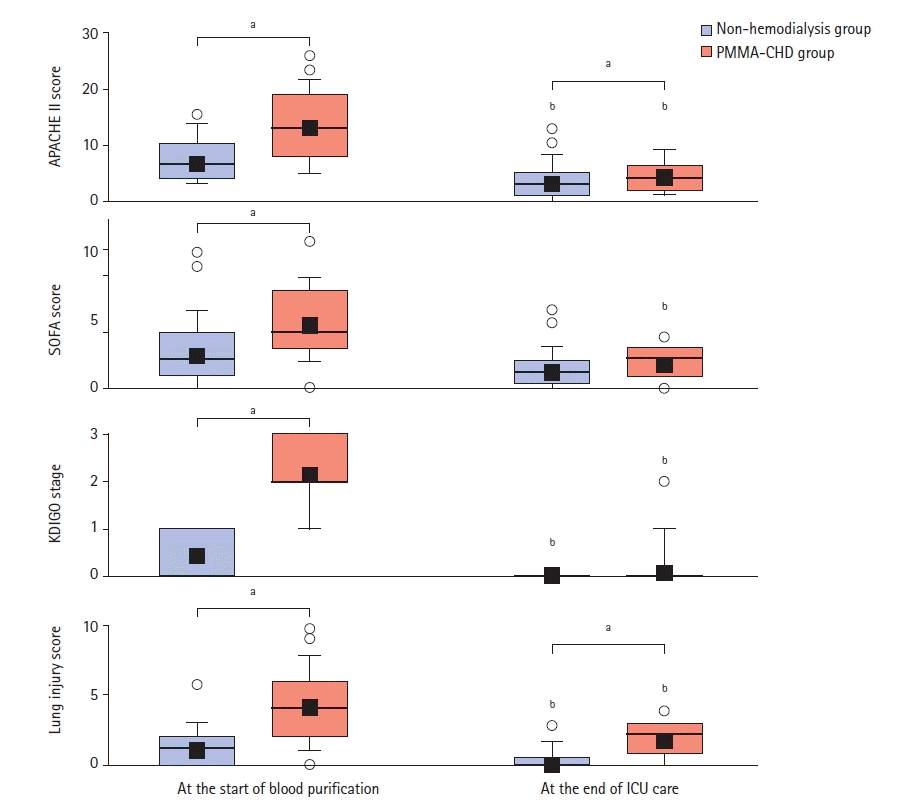
Table 1.
| Variable | Without hemodialysis | PMMA-CHD | P-value |
|---|---|---|---|
| Number of patients | 28 | 26 | - |
| Age (yr) | 50.5±17.0 | 52.1±17.4 | 0.890 |
| Sex (male:female) | 19:9 | 16:10 | 0.777 |
| Body mass index (kg/m2) | 23.7±4.4 | 25.0±3.3 | 0.250 |
| Basic disorder | |||
| Alcohol abuse | 18 | 17 | |
| Cholelithiasis | 6 | 3 | |
| Diabetic hyperglycemia | 1 | 1 | |
| Hyperlipidemia | 1 | 0 | |
| Pancreatic cancer | 0 | 1 | |
| Unidentified | 2 | 3 | |
| Serum amylase (IU/L) | 1,510±1,711 | 1,463±1,466 | 0.917 |
| APACHE II score | 7.0±3.8 | 13.2±6.6 | <0.001 |
| SOFA score | 2.7±2.6 | 4.9±2.7 | 0.002a |
| Balthazar CTSI score | 5.8±1.3 | 6.7±1.6 | 0.056 |
| Japanese enhanced CT grade | 2.7±0.5 | 2.5±0.5 | 0.110 |
| KDIGO stage | 0.5±0.6 | 2.3±0.7 | <0.001a |
| Lung injury score | 1.2±1.4 | 4.3±2.6 | <0.001a |
| Artificial ventilation | 2 (7.1) | 16 (61.5) | <0.001a |
| Hours of starting CHDb | 24.6±18.2 | ||
| Total days of blood purification | 5.0±4.0 | ||
| Days of ICU care | 4.2±3.3 | 9.2±6.1 | <0.001a |
| %Mortality predicted by APACHE II score | 9.4±6.7 | 27.9±31.7 | 0.001a |
| 28-Day survival (%mortality) | 27 (3.6) | 26 (0) | 0.491 |
| Total hospitalization days | 15.6±6.8 | 30.2±22.1 | 0.491 |
| Survivor at discharge (%mortality) | 26 (7.1) | 24 (7.7) | 0.723 |
Values are presented as mean±standard deviation or number (%) unless otherwise indicated.
PMMA-CHD: continuous hemodialysis with a polymethylmethacrylate membrane hemofilter; APACHE: Acute Physiology and Chronic Health Evaluation; SOFA: Sequential Organ Failure Assessment; CTSI: computed tomography (CT) severity index; KDIGO: Kidney Disease: Improving Global Outcome; ICU: intensive care unit.
Table 2.
| Variable | Non-ventilation | Ventilation | P-value |
|---|---|---|---|
| Number of patients | 10 | 16 | - |
| Age (yr) | 43.0±17.8 | 51.5±17.8 | 0.463 |
| Sex (male:female) | 6:4 | 10:6 | 0.747 |
| Body mass index (kg/m2) | 24.0±3.4 | 25.6±3.2 | 0.126 |
| Serum amylase (IU/L) | 1,187±1,426 | 1,635±1,510 | 0.363 |
| APACHE II score | 11.7±7.0 | 15.0±6.4 | 0.140 |
| SOFA score | 4.7±3.0 | 5.3±2.3 | 0.509 |
| Balthazar CTSI score | 6.2±1.1 | 7.5±1.6 | 0.029a |
| Japanese enhanced CT grade | 2.5±0.5 | 2.4±0.5 | 0.760 |
| KDIGO stage | 2.3±0.9 | 2.3±0.7 | 0.906 |
| Lung injury score | 2.4±1.7 | 6.6±1.6 | <0.001a |
| Day of artificial ventilation | - | 7.2±4.4 | |
| Total days of blood purification | 2.2±1.4 | 6.7±4.3 | <0.001a |
| Day of ICU care | 6.4±4.3 | 12.5±6.7 | 0.007a |
| %Mortality predicted by APACHE II score | 28.9±33.4 | 27.4±31.8 | 0.771 |
| 28-Day survival (%mortality) | 10 (0) | 16 (0) | - |
| Total hospitalization days | 24.4±17.3 | 33.8±25.0 | 0.342 |
| Survivor at discharge (% mortality) | 9 (10.0) | 15 (6.2) | 1.000 |
Values are presented as mean±standard deviation.
PMMA-CHD: continuous hemodialysis with a polymethylmethacrylate membrane hemofilter; APACHE: Acute Physiology and Chronic Health Evaluation; SOFA: Sequential Organ Failure Assessment; CTSI: computed tomography (CT) severity index; KDIGO: Kidney Disease: Improving Global Outcome; ICU: intensive care unit.
Table 3.
| Variable | Patient without intermittent HDF | Patient requiring intermittent HDF | P-value |
|---|---|---|---|
| Number of patients | 19 | 7 | - |
| Age (yr) | 51.6±16.7 | 53.3±20.6 | 0.908 |
| Sex (male:female) | 12:7 | 4:3 | 0.779 |
| Body mass index (kg/m2) | 24.8±3.0 | 25.7±4.1 | 0.525 |
| Serum amylase (IU/L) | 850±989 | 1,286±960 | 0.126 |
| APACHE II score | 13.6±7.6 | 12.1±2.7 | 0.977 |
| SOFA score | 5.2±2.9 | 4.0±1.6 | 0.350 |
| Balthazar CTSI score | 6.2±1.5 | 7.9±1.5 | 0.018a |
| Japanese enhanced CT grade | 2.4±0.5 | 2.6±0.5 | 0.504 |
| KDIGO stage | 2.2±0.8 | 2.4±0.5 | 0.593 |
| Lung injury score | 3.7±2.6 | 6.0±1.7 | 0.031a |
| Artificial ventilation | 9 (47.7) | 7 (100) | 0.023a |
| Times of intermittent HDF (number) | 4.1±1.6 | ||
| Total days of blood purification | 3.3±2.1 | 9.9±4.3 | <0.01a |
| Day of ICU care | 7.1±4.7 | 13.9±3.6 | 0.006a |
| %Mortality predicted by APACHE II score | 32.5±36.2 | 15.6±6.1 | 0.977 |
| 28-Day survival (%mortality) | 19 (0) | 7 (0) | - |
| Total hospitalization days | 29.6±19.2 | 31.7±31.6 | 0.839 |
| Survivor at discharge (%mortality) | 17 (10.5) | 7 (0) | 1.000 |
Values are presented as mean±standard deviation unless otherwise indicated.
PMMA-CHD: continuous hemodialysis with a polymethylmethacrylate membrane hemofilter; HDF: hemodiafiltration; APACHE: Acute Physiology and Chronic Health Evaluation; SOFA: Sequential Organ Failure Assessment; CTSI: computed tomography (CT) severity index; KDIGO: Kidney Disease: Improving Global Outcome; ICU: intensive care unit.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download