Abstract
Background
In critically ill patients, the most common manifestation of brain dysfunction is delirium, which is independently associated with higher morbidity and mortality. While electrolyte imbalance is one of the precipitating factors, the impact of hypomagnesemia on the incidence of delirium remains unknown.
Methods
We retrospectively analyzed patients admitted to the medical intensive care unit (ICU) of a tertiary referral center between January and June 2020. Patients with ICU stay ≥48 hours and aged 40–85 years were included. The primary outcome was cumulative incidence of delirium in the ICU. Patients were divided into two groups based on serum magnesium level at ICU admission. Multivariable Cox proportional hazards regression analysis was performed, and covariates were selected using the least absolute shrinkage and selection operator (LASSO) method.
Results
A total of 109 patients included 43 (39.4%) women and had a median age of 69.0 years (interquartile range [IQR], 60.0–76.0 years). The median magnesium level was 1.7 mg/dl (IQR, 1.5–1.9 mg/dl), and the cumulative incidence of delirium was 32.1% (35 patients). Hypomagnesemia was independently associated with delirium (adjusted hazard ratio [aHR], 2.12; 95% confidence interval [CI], 1.03–4.38), along with prior use of immunosuppressants (aHR, 3.08; 95% CI, 1.46–6.48) or benzodiazepines (aHR, 4.02; 95% CI, 1.54–10.50), body mass index (aHR, 0.93; 95% CI, 0.84–1.02), and alcohol history (aHR, 1.68; 95% CI, 0.74–3.80).
Delirium is an acute neuropsychological disorder defined as a disturbance of attention awareness and a change in baseline cognition over a short period of time [1,2]. It is the most common manifestation of brain dysfunction in critically ill patients, reported in 30%–80% of intensive care unit (ICU) patients, depending on the institution and ICU environment [3,4]. Delirium is independently associated with higher mortality, longer hospital stays, longer durations of mechanical ventilation, increased risks of reintubation, higher cognitive impairment, and higher costs for ICU patients [3,5-7]. While haloperidol, atypical antipsychotics, and dexmedetomidine are frequently used to treat delirium, no single pharmacological agent has been proven effective, emphasizing the importance of preventing delirium [2].
Delirium is caused by a variety of factors, necessitating a multicomponent approach for prevention. Patients with predisposing factors, such as preexisting dementia or underlying cognitive impairment, alcoholism, smoking, and advanced age, are at high risk of delirium in the presence of precipitating factors such as infection, hypoxia, hypoglycemia, and electrolyte abnormalities [7-10]. Aspects of ICU care and the environment surrounding the patients, such as use of benzodiazepines, physical restraints, immobilization, indwelling catheters, and absence of visible daylight, also contribute to the development of delirium and are often more modifiable than the predisposing and precipitating factors of the host.
Among the factors associated with delirium, correctable electrolyte abnormalities are modifiable predisposing factors [9,11]. Electrolytes are essential components in the human body that maintain the membrane potential of cells, transmit nerve impulses, and sustain intra- and extracellular fluid balance [12,13]. Magnesium is the fourth most abundant electrolyte in the body and is involved in diverse biochemical reactions such as adenosine triphosphate metabolism, muscle contraction and relaxation, blood pressure regulation, neuronal activity, and neurotransmitter release [14,15]. Patients with hypomagnesemia, who account for 20%–65% of all ICU patients, are at a significantly higher risk of prolonged mechanical ventilation and longer ICU stay, ultimately increasing the risk of delirium [14-18]. Consistent with the role of magnesium, neurological manifestations such as convulsions and coma, have been reported to be associated with hypomagnesemia [16,18]. Although it is reasonable to postulate a similar relationship between hypomagnesemia and delirium, few studies have examined this possible association. Therefore, in this study, we investigated the impact of hypomagnesemia on the incidence of delirium in critically ill, middle-aged, and older adult patients admitted to the medical ICU.
The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-2012-155-1183), and the requirement for written informed consent was waived because of the retrospective study design.
We retrospectively analyzed patients admitted to the medical ICU at Seoul National University Hospital between January 1, 2020 and June 30, 2020. Patients ≥40 years of age and ≤85 years of age who stayed in the ICU for more than 2 days were included in the analysis. Those who were transferred from other ICUs, had acute brain injury or overt neurologic or psychiatric disorders, or had no record of serum magnesium level were excluded. When patients were admitted to the ICU multiple times during the study period, only the first admission was included in the analysis. Several patients who were analyzed in this study were included in our previous report [19].
Demographic and clinical factors potentially associated with delirium were collected at the time of admission to the ICU, including age, sex, body mass index (BMI), smoking history and intensity, alcohol use, baseline serum magnesium level, reason for ICU admission (respiratory, cardiogenic, renal, and septic), and relevant comorbidities such as cerebrovascular diseases and cognitive disorders. Details on the use of antipsychotics, opioids, and hypnotics before ICU admission were recorded. The severity of illness at ICU admission was assessed using the Acute Physiology and Chronic Health Evaluation (APACHE) II score, Simplified Acute Physiology Score (SAPS) II, and Sequential Organ Failure Assessment score. The use of renal replacement therapy and mechanical ventilation, ventilator-free days, duration of mechanical ventilation, length of stay in the ICU, length of hospital stay, mortality in the ICU, and 28-day mortality were collected.
Each patient’s level of sedation and agitation was monitored by trained bedside duty nurses six times per day using the Richmond agitation sedation scale, and the patient was screened for delirium once per day using the Confusion Assessment Method for the ICU (CAM-ICU) [20]. Delirium, the primary outcome of this study, was diagnosed collectively based on the presence of one or more of the following conditions: positive CAM-ICU, confirmation by a consulting psychiatrist, administration of antipsychotics specifically for management of delirium, and clinical diagnosis by the attending physician.
A sample size of 105 patients was determined to provide 85% power using a one-sided alpha (α) level of 0.05. Patient characteristics at ICU admission were summarized as counts and proportions for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. The preliminary analysis compared the factors between patients with and without hypomagnesemia using the Pearson’s chi-square test or Fisher’s exact test for categorical variables and the Student t-test or Mann-Whitney U-test for continuous variables where appropriate. Hypomagnesemia was defined as a serum magnesium concentration less than 1.7 mg/dL. The Kaplan-Meier method was used to delineate the cumulative incidence rates. We examined the relationship between hypomagnesemia and the primary outcome using a Cox proportional hazards regression model. Covariates were selected using the least absolute shrinkage and selection operator (LASSO) method for multivariable analysis among the clinical variables. All analyses were conducted using R software (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria), and all the tests were two-sided with an α of 0.05 to determine statistical significance.
During the study period, 208 patients were admitted to the medical ICU, of whom 109 were included in the analysis (Figure 1). Among the 99 excluded patients, 51 had a less than 48 hour length of stay in the ICU, seven were transferred from other ICUs (surgical, emergency, or coronary care units), 16 were previously admitted to the medical ICU during the study period, two had no account of baseline serum magnesium level, and 23 did not meet the age criteria. The median age was 69 years (IQR, 60–76 years), and 43 patients (39.4%) were women. The median concentration of serum total magnesium was 1.7 mg/dl (IQR, 1.5–1.9 mg/dl). When grouped according to baseline serum magnesium level, there were 46 and 63 patients with hypomagnesemia and normomagnesemia, respectively. While age, sex, BMI, alcohol and smoking history, comorbidities, and medication history were comparable, fewer patients in the hypomagnesemia group were admitted because of a cardiogenic cause (3 [6.5%] vs. 15 [23.8%], P=0.032) or requirement of renal replacement therapy (12 [26.1%] vs. 30 [47.6%], P=0.037). The hypomagnesemia group also exhibited a lower SAPS II median score (41.5 [28.0–49.0] vs. 45.0 [36.5–65.0], P=0.016). The detailed demographic and clinical characteristics of the study patients are summarized in Table 1.
In this study cohort, the cumulative incidence of delirium was 32.1% (35 patients), and the median delirium- or coma-free day was 1.0 days (IQR, 0.0–3.0 days) (Table 2). The cumulative incidence of delirium (18% [28.6%] vs. 17% [37.0%], P=0.47) and the median delirium- or coma-free days (2.0 [0.0–4.0] vs. 1.0 [0.0–3.0], P=0.14) did not differ between the hypo- and normomagnesemia groups. There was no significant difference in the cumulative hazard of delirium between the two groups (Figure 2). We did not observe statistical differences between the hypo- and normomagnesemia groups in number of ventilator-applied patients, median duration of mechanical ventilation, and median ventilator-free days. ICU mortality and 28-day mortality were comparable between the two groups.
Next, we examined the factors associated with the incidence of delirium in the ICU (Table 3). In univariate analysis, prior use of immunosuppressants (hazard ratio [HR], 2.82; 95% CI, 1.20–6.66) or benzodiazepines (HR, 3.45; 95% confidence interval [CI], 1.01–11.79) was significantly associated with an elevated risk of delirium incidence. Among these factors, the LASSO method indicated that hypomagnesemia, BMI, alcohol history, and prior use of immunosuppressants and/or benzodiazepines were associated with incidence of delirium. The adjusted hazard ratio (aHR) of hypomagnesemia from multivariable Cox proportional hazards regression analysis using these selected variables was 2.12 (95% CI, 1.03–4.38); that of immunosuppressants was 3.08 (95% CI, 1.46–6.48); and that of benzodiazepines was 4.02 (95% CI, 1.54–10.50). The association with BMI (aHR, 0.93; 95% CI, 0.84–1.02) and alcohol history (aHR, 1.68; 95% CI, 0.74–3.80) was not statistically significant.
We retrospectively examined the relationship between hypomagnesemia and incidence of delirium in patients aged between 40 and 85 years who spent 2 or more consecutive days in the medical ICU. Multivariable Cox proportional hazards regression analysis adjusted for BMI, prior use of immunosuppressants, benzodiazepines, and alcohol demonstrated that hypomagnesemia was independently associated with increased risk of delirium.
Magnesium functions as a cofactor in more than 300 enzyme systems; regulates diverse biochemical metabolism; maintains the stabilization of cellular membranes, proteins, and nucleic acid synthesis; and participates in cellular timekeeping [21-23]. Magnesium is widely known to exhibit a neuroprotective effect in preclinical models and has been tested for its role in acute stroke and subarachnoid hemorrhage [24,25], although the results are controversial. Its association with sleep disorders and sedation in the ICU has been reported in other studies [23,26]. The latter study involved a randomized controlled trial of addition of intravenous magnesium to the traditional sedatives in the ICU and found that patients who received magnesium had decreased use of midazolam and additional analgesics without side effects. While these results suggest that magnesium level is related to delirium, there have been no definitive studies to date.
In our study, patients with hypomagnesemia exhibited a two-fold increased risk of delirium compared to those without hypomagnesemia. One possible explanation for this finding is the reduced neuroprotective effect in patients with hypomagnesemia, as mentioned above. Specifically, deficiency of magnesium, which is a vital element for establishing the electrical potential across cell membranes, activating enzymes, and regulating calcium metabolism, can induce unwanted dysfunction in neuronal activities [15]. In addition, the analgesic properties of magnesium through inhibitory effects on N-methyl-D-aspartate receptors can be reduced in hypomagnesemia, leading to increased vulnerability to pain in the ICU [15]. Furthermore, hypomagnesemia can increase the risk of specific conditions and procedures that are known precipitating factors for delirium. Previous studies have shown a more frequent need for ventilatory support, prolonged duration of mechanical ventilation, and more frequent association with sepsis in critically ill patients with hypomagnesemia [27]. Although not statistically significant, patients with hypomagnesemia exhibited fewer median ventilator-free days (1.0 vs. 3.0 days, P=0.09) and longer median duration of mechanical ventilation (3.0 vs. 4.0, P=0.55) despite less severe illness at baseline (SAPS II score, 41.5 vs. 45.0; P=0.016).
Our finding that hypomagnesemia is associated with increased risk of delirium in the ICU is important and has meaningful clinical implications in the diagnosis and management of delirium. Delirium in the ICU is multifaceted and can present as hyperactive, hypoactive, or mixed hyperactive and hypoactive states. Although many validated screening tools such as the CAM-ICU have been developed, delirium detection remains demanding, particularly for the hypoactive state. Our findings could help improve the detection rate of delirium by guiding intensivists and nursing staff to carefully examine patients with hypomagnesemia. In addition, while there is currently no single pharmacological agent that has been proven to be effective in the prevention and management of delirium, it would be interesting to determine whether the supplementation of magnesium in patients with hypomagnesemia could lead to delirium prevention and/or control, meriting a future well-designed randomized controlled trial.
This study has several limitations. This was a retrospective, observational, proof-of-concept study conducted in a single medical ICU. Prospective, multicenter studies including non-medical patients are necessary before the results of our findings can be applied. In addition, since only free magnesium is biologically active [21], the use of serum total concentration of magnesium in our study might have overestimated the number of patients with hypomagnesemia. Future studies adopting magnesium tolerance tests or ionized magnesium-level measurements might provide additional information. Furthermore, only the baseline magnesium concentration was used for analysis because of data availability. It would be interesting to examine the longitudinal changes in magnesium concentration between patients with and without delirium. Although there were no distinct differences in baseline characteristics between patients with and without hypomagnesemia, and all the relevant factors selected by the LASSO method were taken into consideration for multivariable analysis, other factors that affect magnesium concentration, such as dietary intake, might have confounded the results.
In conclusion, in critically ill patients 40–85 years of age, hypomagnesemia increases the risk of delirium by more than two-fold compared to that in patients with normal magnesium level. The initial serum magnesium level upon ICU admission is a potential predictive marker of delirium in these patients. Therefore, more attention to hypomagnesemia is warranted.
▪ In a multivariable Cox proportional hazards regression analysis of critically ill patients aged 40–85 years in the medical intensive care unit (ICU), hypomagnesemia was independently associated with the cumulative incidence of delirium adjusted for body mass index, prior use of immunosuppressants and/or benzodiazepines, and alcohol history.
▪ Hypomagnesemia increases the risk of delirium by more than two-fold compared to patients with normal magnesium level.
▪ Hypomagnesemia is a possible predictive marker of delirium in medical ICU settings, warranting greater attention.
REFERENCES
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington (DC): American Psychiatric Association; 2013.
2. Stollings JL, Kotfis K, Chanques G, Pun BT, Pandharipande PP, Ely EW. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med. 2021; 47:1089–03.
3. Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004; 291:1753–62.
4. Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015; 350:h2538.
5. Vasilevskis EE, Chandrasekhar R, Holtze CH, Graves J, Speroff T, Girard TD, et al. The cost of ICU delirium and coma in the intensive care unit patient. Med Care. 2018; 56:890–7.
6. Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004; 32:2254–9.
7. Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007; 33:66–73.
8. Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009; 13:R77.
9. Sonneville R, de Montmollin E, Poujade J, Garrouste-Orgeas M, Souweine B, Darmon M, et al. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med. 2017; 43:1075–84.

11. Zieschang T, Wolf M, Vellappallil T, Uhlmann L, Oster P, Kopf D. The Association of hyponatremia, risk of confusional state, and mortality. Dtsch Arztebl Int. 2016; 113:855–62.

13. Shrimanker I, Bhattarai S. Electrolytes. Treasure Island: StatPearls Publishing;2021.
14. Cirik MÖ, Kilinç M, Doğanay GE, Ünver M, Yildiz M, Avci S. The relationship between magnesium levels and mortality in the respiratory intensive care unit. Medicine (Baltimore). 2020; 99:e23290.

15. Panahi Y, Mojtahedzadeh M, Najafi A, Ghaini MR, Abdollahi M, Sharifzadeh M, et al. The role of magnesium sulfate in the intensive care unit. EXCLI J. 2017; 16:464–82.
16. Fairley J, Glassford NJ, Zhang L, Bellomo R. Magnesium status and magnesium therapy in critically ill patients: a systematic review. J Crit Care. 2015; 30:1349–58.

17. Karnik ND, Gupta AV. Hypomagnesemia in ICU. J Assoc Physicians India. 2016; 64:11–3.
18. Noronha JL, Matuschak GM. Magnesium in critical illness: metabolism, assessment, and treatment. Intensive Care Med. 2002; 28:667–79.

19. Lee HJ, Bae E, Lee HY, Lee SM, Lee J. Association of natural light exposure and delirium according to the presence or absence of windows in the intensive care unit. Acute Crit Care. 2021; 36:332–41.

20. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990; 113:941–8.

22. Cao Y, Zhen S, Taylor AW, Appleton S, Atlantis E, Shi Z. Magnesium Intake and sleep disorder symptoms: findings from the Jiangsu nutrition study of Chinese adults at five-year follow-up. Nutrients. 2018; 10:1354.

23. Kumar S, Jain S, Agrawal S, Honmode A. Impact of serum magnesium levels in critically ill elderly patients: a study in a rural teaching hospital. J Clin Gerontol Geriatr. 2016; 7:104–8.
24. Dorhout Mees SM, Algra A, Vandertop WP, van Kooten F, Kuijsten HA, Boiten J, et al. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomised placebo-controlled trial. Lancet. 2012; 380:44–9.

25. Saver JL, Starkman S, Eckstein M, Stratton SJ, Pratt FD, Hamilton S, et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med. 2015; 372:528–36.

26. Altun D, Eren G, Cetingok H, Hergünsel GO, Çukurova Z. Can we use magnesium for sedation in the intensive care unit for critically ill patients: is it as effective as other sedatives? Agri. 2019; 31:86–92.
27. Limaye CS, Londhey VA, Nadkart MY, Borges NE. Hypomagnesemia in critically ill medical patients. J Assoc Physicians India. 2011; 59:19–22.
Figure 1.
Flowchart of the patients included in the study. MICU: medical intensive care unit; ICU: intensive care unit.
Figure 2.
Cumulative hazard plot of delirium in the intensive care unit stratified by magnesium (Mg) level.
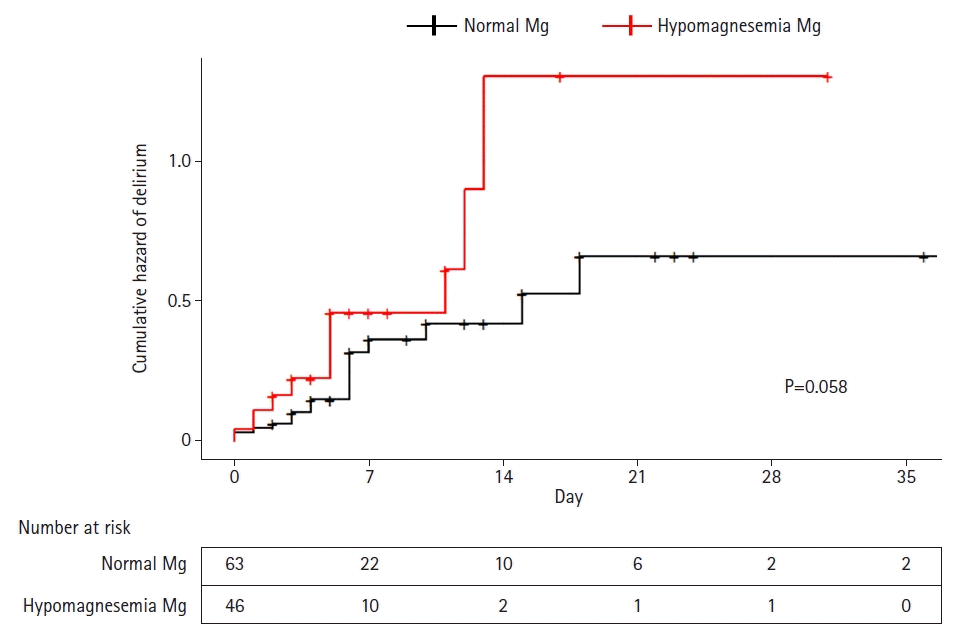
Table 1.
Baseline patient characteristics by Mg level
Values are presented as median (interquartile range) or number (%).
Mg: magnesium; BMI: body mass index; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; ICU: intensive care unit; APACHE: Acute Physiology and Chronic Health Evaluation; SOFA: Sequential Organ Failure Assessment; SAPS: Simplified Acute Physiology Score.
Table 2.
Outcomes of patients by Mg level
Table 3.
Univariable and multivariable Cox proportional hazards regression




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download