Abstract
Background
Phlebitis-associated peripheral infusion of intravenous amiodarone is common in clinical practice, with an incidence between 5% and 65%. Several factors, including drug concentration, catheter size, and in-line filter used, are significantly associated with phlebitis occurrence. We performed a retrospective propensity score-matched analysis to find out whether in-line filter will reduce the incidence of amiodarone-induced phlebitis (AIP) in high concentration of amiodarone infusion compared to low concentration without in-line filter.
Methods
Clinical records of all patients who required intravenous amiodarone infusion for cardiac arrhythmias, between January 2017 to December 2019 were retrieved. The incidence of AIP was recorded and subsequently compared among high concentration (2 mg/ml) with an in-line filter and low concentration (1.5 mg/ml) infusion without an in-line filter after a 1 to 2 propensity score matched.
Results
The data indicated that among the 214 cases of amiodarone infusion collected, 28 cases used an in-line filter with high concentration while 186 cases received a low concentration of amiodarone infusion without an in-line filter. After 1:2 propensity score matching, the incidence of phlebitis in the high concentration with in-line filter group was significantly higher than the low concentration without in-line filter group (28.6% vs. 3.6%, P<0.01).
Amiodarone is an anti-arrhythmic agent, that is commonly used in the intensive care unit to control supraventricular, and ventricular arrhythmias, including atrial fibrillation, atrial flutter, and ventricular tachycardia [1]. Although infusion via central venous catheter is recommended to attenuate the incidence of amiodarone-induced phlebitis (AIP), central venous catheterization may be technically difficult in an emergency. Therefore, peripheral intravenous infusion would be an appropriate channel of medication.
AIP is a common adverse reaction of amiodarone infusion, with an incidence between 5%–85%, depending on the specific infusion protocol [2-5]. Phlebitis is the inflammation of the peripheral vein, which results in pain, erythema, and swelling of skin overlying the peripheral vein. Phlebitis is mostly a localized reaction but can progress to systemic inflammation including fever, and hemodynamic changes in severe cases. The severity of AIP is classified by several groups. The Seminal Infusion Nurses Society (INS) Scale and visual phlebitis scale are both divided into 4 severity levels from 0 (no phlebitis) to 4 (severe phlebitis) [4,6].
Theoretically, AIP develops from physical and chemical injury. Physical phlebitis is related to poor catheter insertion and malposition of the peripheral venous catheter. On the other hand, chemical phlebitis is related to the properties of intravenous amiodarone itself, which easily crystallizes after infusion. The amiodarone needle-shaped crystal injures the endothelium and leads to phlebitis development [7]. Therefore, a low concentration of amiodarone and an in-line filter is recommended to reduce the crystallization of amiodarone. The peripheral large vein is also suggested for use in peripheral amiodarone infusion [7]. From a recent systematic review of AIP, the size of the vein and venous catheter, as well as the concentration of amiodarone, are among the risk factors of AIP [8].
In our hospital, the infusion of amiodarone via the peripheral vein is the first-line treatment for controlling cardiac arrhythmias. We prefer a low concentration of amiodarone rather than the high concentration mixture administrated via a peripheral venous catheter. The in-line filter is, however, costly and intermittently unavailable in our place. Therefore, in-line filter is not commonly used with low concentration infusion of amiodarone. Nonetheless, a high concentration of amiodarone could be used for patients who require fluid restriction. To prevent AIP in a high concentration regimen, an in-line filter is also theoretically suggested. In addition, the in-line filter could be used in high-risk patients, for example elderly, difficult venous cannulation, and prolonged infusion. The evidence of in-line filter to prevent AIP in the high concentration of amiodarone infusion via the peripheral venous catheter remains unclear. We, therefore, conducted this retrospective matched analysis to find out whether the administration of the high concentration of continuous amiodarone infusion with in-line filter may reduce the incidence of AIP compared to low concentration infusion.
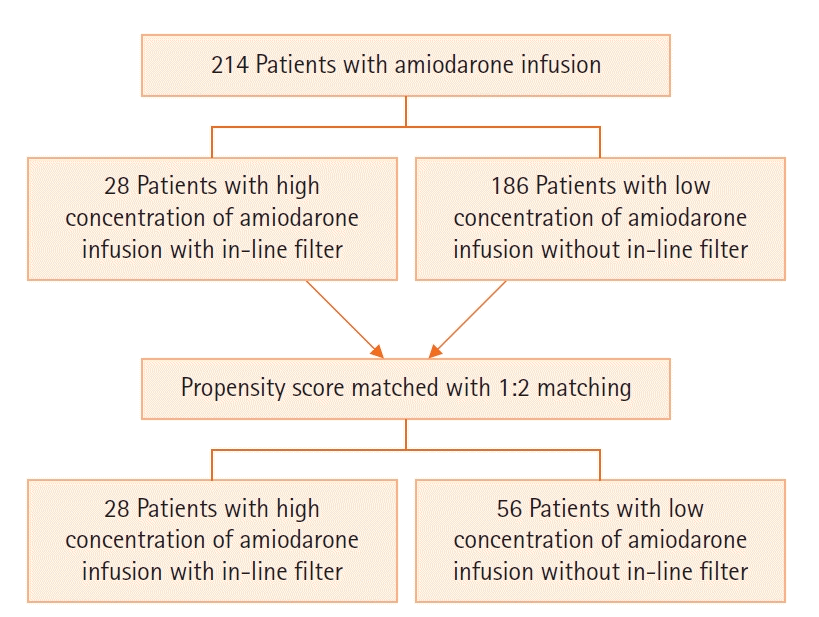
We included patients admitted to the intensive care unit and cardiac care unit between January 2017 to December 2019, who required continuous intravenous amiodarone infusion for cardiac arrhythmias for at least 12 hours (Figure 1). We excluded moribund cases, patients who had central venous catheterization, and postcardiac arrest patients. This study has been approved by Institutional Review Board of Faculty of Medicine, Prince of Songkla University on October10, 2019 with the registration number of REC.62-044-15-7. The inform consent was waived because it is a retrospective study and all personal information were blinded.
Before starting amiodarone infusion, the 24-gauge peripheral venous catheter was inserted into a large peripheral vein such as the antecubital vein, vein at the dorsum of the hand, vein at the forearm, and arm. A clear adhesive dressing was applied to cover the catheter insertion site. Subsequently, 20 ml of normal saline was flushed through the venous catheter by push and pause technique. If there was no leakage, amiodarone infusion was then started.
The amiodarone infusion started with a loading dose and subsequent continuous intravenous infusion. For the peripheral venous catheter infusion, an amiodarone loading concentration of 1.5 mg/ml with a total dose of 150 mg diluted in 100 ml of dextrose 5% in water (D5W) was given within 30 minutes. Subsequently, amiodarone continuous infusion was started with two concentrations regimens, either low concentration or high concentration. The low concentration regimen was an amiodarone concentration of 1.5 mg/ml (900 mg of amiodarone in D5W 600 ml), and the high concentration was a concentration of 2 mg/ml (900 mg of amiodarone in D5W 450 ml). The drug concentration selected was at the discretion of the treating clinician. The in-line filter was applied to all patients receiving a high concentration amiodarone infusion. Amiodarone infusions were continued for at least 24 hours, until ceased by the treating clinician.
The in-line filter with filter media of 0.2 micro millimeters, positively charged Nylon Posidyne membrane (PALL Medical, Port Washington, NY, USA), was used in every case who received a high concentration regimen. The in-line filter was connected to the end of the intravenous fluid delivery tube and the other end was connected to the peripheral venous catheter. From our hospital protocol, the peripheral catheter site was changed every 24 hours in patients who required longer than 24 hours amiodarone infusion to reduce the risk of AIP. Normal saline flushing was performed by the push and pause technique before using the peripheral venous catheter at the new insertion site.
The occurrence of phlebitis was continuously monitored every 1 hour during amiodarone infusion, and every 4 hours at the cease of infusion. Phlebitis was classified into 4 severity levels according to the visual phlebitis scale (Table 1). The present and severity of AIP is evaluated by the nurse and was finally confirmed by the treating physician. The most severe AIP was then collected and recorded for the primary outcome.
The study outcomes are the incidence and severity of phlebitis between the two regimens.
We reported the incidence of AIP in our cohort by number and percentage. Subsequently, we performed univariate analysis to identify to risk factors to AIP. Selected variables that were statistically significant with P<0.1 were then introduced into a forward, stepwise, logistic regression model. Odds ratios (ORs) and their 95% confidence intervals (CIs) were used to identify the independent factors of AIP.
We employed the stepwise, binary logistic regression method to calculate propensity score, using pretreatment patient clinical characteristic that increased the likelihood of receiving a high concentration of amiodarone, including age, sex, types of cardiac arrhythmias, and previous phlebitis. After the propensity score was obtained, the propensity score match cohort between the high concentration of amiodarone with in-line filter group and low concentration of amiodarone without in-line filter group were performed by 1:2 matching with a caliper of score difference of 0.01.
After propensity score matching, the incidence of phlebitis between the groups was compared by chi-square test. The continuous variables were presented as median with minimum and maximum values or mean with standard deviation, depending on data distribution. The categorical variables were presented as numbers and percentages. The comparison of clinical characteristics and outcomes was computed by independent t-test, Mann-Whitney U-test, chi-square test, or Fisher’s exact test as appropriate. A P<0.05 was defined as statistically significant. All statistical analyses were performed using the MedCalc Statistical Software ver. 20.022 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; 2021).
During the study period, 214 patients were admitted to the intensive care unit for cardiac arrhythmia requiring amiodarone infusion. The median age of the selected patients was 73 years (16–98 years) and 61.7% were male. The most common cardiac arrhythmia was atrial fibrillation (80.8%), followed by ventricular tachycardia (17.3%). Twenty-eight cases (13.1%) of amiodarone infusion received high concentration with an in-line filter. Vein at the dorsum of the hand (35.5%) was the most common site of peripheral catheterization, follow by vein at the wrist (18.2%), lower arm (12.6%), cubital fossa (9.8%), and upper arm (8.4%), respectively. Ninety-three percent of cases received amiodarone infusion within 24 hours.
The overall incidence of AIP in our cohort was 8.4% (18/214 patients), with 12 of 18 patients (66.6%) developed only grade 1, 2 AIP severity. According to the low incidence of AIP in our cohort, we performed the univariate analysis of the whole cohort to identify the risk factors of AIP development. There is no significantly difference in clinical characteristics including age, sex, location of peripheral catheter, types of cardiac arrhythmias, previous phlebitis, and skin turgor for the AIP development or severity of AIP. Only the high concentration of amiodarone infusion with in-line filter was significantly related to AIP (Table 2). We also found that the high concentration of amiodarone infusion with in-line filter was an independent factor of AIP with the adjusted odd ratio of 7.35 (95% CI, 2.55–21.21; P=0.002).
After the propensity scores were calculated, the 28 patients in the high concentration with in-line filter group were 1:2 matched to 56 patients in the low concentration group (Table 3). All demographic data were completely matched. Eight cases (28.6%) in the high concentration with in-line filter group developed AIP with 3 of 8 cases (37.5%) were in the grade 3, 4 AIP, while two cases (3.6%) in the low concentration group (P<0.01) developed grade 1, 2 AIP. AIP occurred significantly more delayed in high concentration with in-line filter group than in low concentration group (17.4±6.5 hours vs. 4.5±21 hours, P=0.03).
The current study demonstrated that the overall incidence of AIP after amiodarone infusion was around 8.4%. However, the incidence of AIP was significantly higher in the high concentration with in-line filter group than in the low concentration group (28.6% vs 3.6%, P<0.01), which could imply that in-line filter application with the infusion of amiodarone at a high concentration of 2 mg/ml did not prevent the AIP. Furthermore, the AIP developed at a mean time of 17.4 (standard deviation, 6.5) hours after the start of infusion in the high concentration regimen, which occurred more delay than the low concentration group. Severe AIP (grade 3-4) was around 37.5% in only high concentration with in-line filter group, which no severe AIP occurred in low concentration infusion.
According to the Infusion Nurses Society, the rate of phlebitis from any peripheral administration of intravenous drug should be less than 5% [3,9]. Several studies have reported AIP incidence of around 5% to 65% [1-4,8]. In our cohort, the overall incidence of AIP was 8.4%, although this developed mainly in the high concentration with in-line filter group. However, the low concentration of amiodarone without in-line resulted in the significantly lower incidence of AIP (3.6%), which was lower than the standard value. In addition, the high concentration of amiodarone infusion with in-line filter was only an independent factor for AIP in our cohort. Therefore, the high concentration of amiodarone infusion (2 mg/ml) should not be administered via peripheral venous catheter regardless of in-line filter in term of AIP development.
The risk factors of AIP include high drug concentration, longer infusion time, lower infusion rate, and higher total accumulative dose [7]. The current recommendation from several consensuses suggests the use of a lower concentration of amiodarone, application of the in-line filter, and using central venous infusion [10]. Studies reported that an amiodarone concentration of 1.2 mg/ml resulted in a significantly lower rate of phlebitis than an amiodarone concentration of 1.8 mg/ml [5,11]. Furthermore, a study on in-line filters for amiodarone infusion in the prevention of AIP, documented that AIP rates were significantly lower in the presence of in-line filters (OR, 0.23; 95% CI, 0.15–0.34; P<0.001) [7]. In our study, we did not support the application of in-line filter in high amiodarone concentration to prevent AIP in peripheral venous infusion. Therefore, the administration of high concentration of amiodarone must be administered via central venous catheter instead of peripheral venous catheter. We still uncleared about why the development of AIP in high concentration with in-line filter was more delayed compared to the lower concentration one but suggest the dynamic of drug crystallization could be a reason of our finding [7].
In addition, an integrated team approach with multidisciplinary healthcare providers for close monitoring of AIP development in amiodarone infusion cases, as well as hospital intravenous fluid team, were among the recommendation for AIP prevention [3,10]. However, these modalities are difficult to fulfill in some situations, for example, in an emergency setting where central venous catheterization is not suitable, and in fluid-restricted patients that could not tolerate the large volume of intravenous infusion. Higher drug concentration with add-on in-line filter is another choice for specific patients such as heart failure, renal failure, and patients with clinical fluid overloaded.
In our hospital, we developed an evidence-based amiodarone infusion protocol to prevent AIP as a recommendation. Standard low concentration of amiodarone at 1.5 mg/ml is administered via a peripheral vein, without regular use of in-line filter due to high cost and limited supply of the filter. Niël-Weise et al. [12] recommended that in-line filters should not be generally used in every intravenous infusion, and suggested usage with specific agents with a high incidence of phlebitis. From our matched analysis, the low concentration protocol demonstrated a lower AIP development and mostly in grade 1, 2 AIP, but the higher concentration with in-line filter resulted in AIP incidence higher than the standard acceptable value. We have abandoned the infusion of high concentration of amiodarone via peripheral venous catheter regardless of in-line filter in our hospital and recommended to insert the central venous catheter if the high concentration of amiodarone infusion is required.
However, our study has some limitations. Notwithstanding the propensity score-matched analysis in this study, the limitations of retrospective studies could not be avoided. The propensity score match analysis could not eliminate the unmeasured confounding variables, like randomized control study [13]. In addition, the study sample size was small, the application of our result must be cautioned. Although our study might not be generally applied across institutes, our finding may confirm the safety of the protocolized low concentration of amiodarone infusion in peripheral vein without in-line filter and emphasize the risk of higher amiodarone concentration of 2 mg/ml in AIP development despite the application of an in-line filter. The high concentration of amiodarone must be administered via the central venous catheter to prevent AIP.
The incidence of AIP was high for the high amiodarone concentration infusion with in-line filter protocol. From the current matched analysis, the high concentration of amiodarone infusion at 2 mg/ml with an in-line filter could not prevent AIP and the incidence was higher than the acceptable value. From our finding, we strongly suggested that the administration of amiodarone with a high concentration (2 mg/ml) should be infused via central venous catheter to prevent AIP development.
▪ Amiodarone-induced phlebitis is common and associated with morbidity.
▪ The administration of high concentration of amiodarone of 2 mg/ml with an in-line filter did not reduce phlebitis. Central venous catheterization for a high concentration of amiodarone is recommended.
▪ Amiodarone concentration of 1.5 mg/ml is safe and can prevent phlebitis.
ACKNOWLEDGMENTS
The author would like to sincerely thanks Mr. Ozioma Forstinus Nwabor, Department of Internal Medicine, Faculty of Medicine, Prince of Songkla University for the English language correction.
REFERENCES
1. Hilleman DE, Hansen JM, Mohiuddin SM. Amiodarone-induced infusion phlebitis. Clin Pharm. 1987; 6:364–367.
2. Boyce BA, Yee BH. Incidence and severity of phlebitis in patients receiving peripherally infused amiodarone. Crit Care Nurse. 2012; 32:27–34.
3. Dixon HA, Hort AL, Wright CM. Amiodarone-induced phlebitis remains an issue in spite of measures to reduce its occurrence. J Vasc Access. 2019; 20:786–7.
4. Hannibal GB. Peripheral phlebitis related to amiodarone infusion. AACN Adv Crit Care. 2016; 27:465–71.
5. Norton L, Ottoboni LK, Varady A, Yang-Lu CY, Becker N, Cotter T, et al. Phlebitis in amiodarone administration: incidence, contributing factors, and clinical implications. Am J Crit Care. 2013; 22:498–505.
6. Sharifi-Ardani M, Yekefallah L, Asefzadeh S, Nassiri-Asl M. Efficacy of topical chamomile on the incidence of phlebitis due to an amiodarone infusion in coronary care patients: a double-blind, randomized controlled trial. J Integr Med. 2017; 15:373–8.
7. Oragano CA, Patton D, Moore Z. Phlebitis in intravenous amiodarone administration: incidence and contributing factors. Crit Care Nurse. 2019; 39:e1–12.
8. Hilleman DE, Spinler SA. Conversion of recent-onset atrial fibrillation with intravenous amiodarone: a meta-analysis of randomized controlled trials. Pharmacotherapy. 2002; 22:66–74.
9. Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006; 29(Suppl 1):S1–92.
10. Murphy K, Murphy J, Fischer-Cartlidge E. Reducing the Incidence of amiodarone-related phlebitis through utilization of evidence-based practice. Worldviews Evid Based Nurs. 2020; 17:385–92.
11. Mowry JL, Hartman LS. Intravascular thrombophlebitis related to the peripheral infusion of amiodarone and vancomycin. West J Nurs Res. 2011; 33:457–71.
12. Niël-Weise BS, Stijnen T, van den Broek PJ. Should in-line filters be used in peripheral intravenous catheters to prevent infusion-related phlebitis? A systematic review of randomized controlled trials. Anesth Analg. 2010; 110:1624–9.
Table 1.
Phlebitis scale
Table 2.
Clinical characteristics between the patient with and without amiodarone-induced phlebitis
Table 3.
Patient characteristics and outcomes before and after propensity score-matched analysis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download