Abstract
Background
Prediction of intensive care unit (ICU) mortality in traumatic brain injury (TBI), which is a common cause of death in children and young adults, is important for injury management. Neuroinflammation is responsible for both primary and secondary brain injury, and C-reactive protein-albumin ratio (CAR) has allowed use of biomarkers such as procalcitonin (PCT) in predicting mortality. Here, we compared the performance of CAR and PCT in predicting ICU mortality in TBI.
Methods
Adults with TBI were enrolled in our study. The medical records of 82 isolated TBI patients were reviewed retrospectively.
Results
The mean patient age was 49.0 ± 22.69 years; 59 of all patients (72%) were discharged, and 23 (28%) died. There was a statistically significant difference between PCT and CAR values according to mortality (P<0.05). The area under the curve (AUC) was 0.646 with 0.071 standard error for PCT and 0.642 with 0.066 standard error for CAR. The PCT showed a similar AUC of the receiver operating characteristic to CAR.
Conclusions
This study shows that CAR and PCT are usable biomarkers to predict ICU mortality in TBI. When the determined cut-off values are used to predict the course of the disease, the CAR and PCT biomarkers will provide more effective information for treatment planning and for preparation of the family for the treatment process and to manage their outcome expectations.
Traumatic brain injury (TBI), which is known as a silent epidemic, is a common cause of disability and death in children and adults worldwide [1]. Therefore, estimation of the damage caused by TBI is important for the treatment plan in terms of preparing families for possible situations. The Glasgow Coma Score (GCS) has been used for many years to evaluate the neurological status of brain injury patients and to determine the severity of TBI [2]; however, it has limited use in predicting mortality [3].
Current scoring systems used to predict mortality in TBI include the Acute Physiology and Chronic Health Evaluation (APACHE) II system and a combination of the GCS and other physiological parameters. The Inflammation, Nutrition, Consciousness, Neurological function, and Systemic function scale [4] is a new TBI-specific scoring system that gives accurate results; however, it requires time to obtain the data and requires an application to perform the calculation. Therefore, new markers in predicting mortality in TBI continue to be investigated.
Many metabolic, physical, and biochemical events that occur after TBI initiate neuroinflammation, which affects mortality. Importantly, the mechanisms of injury include apoptosis of neural cells, blood brain barrier dysfunction, and ischemia. TBI activates the microglia, induces cytokine production in the brain, and causes migration of peripheral immune cells to the damaged area. Microglia are the first responders to brain injury, and co-activation with astrocytes might be responsible for neuroinflammation and long-term damage [5].
C-reactive protein-albumin ratio (CAR), which has been proven as useful in predicting mortality in diseases such as sepsis, hepatocellular carcinoma, and pancreatic cancer, has started to be used as a biomarker in TBI [6]. Neuroinflammation, which occurs when neural components (glial cells, axons, neurons) are damaged by mechanical forces, is responsible for both primary and secondary brain damage [5]. Therefore, CAR, an inflammation-based score, can be useful in predicting mortality in TBI.
Procalcitonin (PCT) is the 116-amino acid polypeptide prohormone of calcitonin, and its presence has been identified in many tissues, including the brain [7]. While there is no significant amount in serum in healthy individuals, serum PCT level is elevated in conditions such as bacterial sepsis, head trauma, and severe trauma [8]. In this study, we aimed to compare the performance of CAR and PCT in predicting mortality in TBI.
The ethical committee for clinical research of Muğla Sıtkı Koçman University approved this study on February 3, 2021 (No. 3/VIII). Adults with TBI who were admitted to the Anesthesiology intensive care unit (ICU) of Muğla Research and Training Hospital from 2019 to 2020 were enrolled in our study. Patient consent was waived because the local ethics committee approved the retrospective study. The medical records of 91 isolated TBI patients were reviewed retrospectively. Patients younger than 18 years, pregnant women, and those with missing data were excluded from the study (Figure 1). The following data were obtained from the medical records: age, gender, comorbidity, intubation, mechanical ventilation duration, number of days of intensive care hospitalization, surgery, ICU exit status (discharge-exitus), and vasopressor/inotrope need within the first 24 hours. In addition, GCS, APACHE II score, CRP, albumin, and PCT values of the patients within the first 24 hours of admission to ICU were noted.
Statistical analysis was performed using the IBM SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean±standard deviation and categorical variables are expressed as percentage. Continuous variables were analyzed using Student t-tests for normally distributed variables, and Mann-Whitney U-tests for non-normally distributed variables. Categorical variables were analyzed using Pearson’s chi-Square test analysis and Fisher’s exact tests, when appropriate. In all tests, a P-value below 0.05 was considered statistically significant.
Receiver operating characteristics curve (ROC) analysis was used to determine the predictive power of the APACHE II, GCS, CAR, and PCT variables. When a significant cut-off value was observed, the sensitivity, specificity, and positive and negative predictive values were presented. When evaluating the area under the curve (AUC), a 5% type-I error level was used to accept a statistically significant predictive value of the test variables. Calibration of the prognostic models, defined as the accuracy of the estimated mortality rate, was assessed using the Hosmer-Lemeshow goodness-of-fit test, standardized mortality rate, and calibration curves.
Possible factors identified via univariate analyses were further analyzed using the Cox regression analysis with backward selection to determine independent predictors of survival. The Kaplan-Meier survival estimates were calculated. Among correlated factors with similar effects on survival, only those with clinical significance were included. The proportional hazards assumption and model fit were assessed by means of residual (Schoenfeld and Martingale) analysis. A 5% type-I error level was used to infer statistical significance.
The G-power program was used in power calculations. Post-treatment power levels for PCT and CAR were calculated by referencing the statistical results outlined in Table 1. The AUC value of PCT was 0.646±0.071 and the AUC value of CAR was 0.642±0.066. The standard AUC value to be tested was 0.5 and the power level calculated for n=82 (23 deaths, 59 survivals) was 99.6%.
A total of 91 patients was identified for the study; however, five patients were excluded due to lack of medical data and four patients were excluded because they were younger than 18 years. Statistical analysis was conducted on 82 patients. The mean age of the patients was 49.0±22.69 years, 56 patients (68.3%) were male and 26 (31.7%) were female. A total of 43 patients (52.4%) had at least one comorbidity, while 18 (22%) required a vasopressor/inotrope. A total of 59 patients (72%) was discharged and 23 (28%) died (Table 2).
There was a statistically significant difference between PCT and CAR values according to mortality (P=0.041 and P=0.047 respectively). Therefore, we calculated cut-off points for PCT, CAR, and GCS according to mortality by ROC analysis. The AUC was 0.646 with 0.071 standard error for PCT and 0.642 with 0.066 standard error for CAR. The PCT showed similar AUC of the receiver operating characteristic (AUROC) score to CAR; however, the AUROC of GCS was lower than that of PCT and CAR (AUROC: 0.614, 0.646, 0.642, respectively) (Figure 2 and Table 1).
Results determined a PCT cut-off point of 1.16, with a sensitivity of 65.2% and specificity of 66.1%. In addition, a CAR cut-off point of 0.32 was identified, with a sensitivity of 61% and specificity of 60.09% (Table 3). Cox regression analysis was performed to determine the factors affecting ICU mortality. CAR was compared with the outcome variable (death or survival) of PCR and GCS. PCT and CAR pose a 1.040 (95% CI, 1.004–1.077) and 16.755 (95% CI, 1.606–174.876) times higher risk for mortality, respectively (Table 4).
TBI is a heterogeneous injury that occurs due to external mechanical force and can result in temporary or permanent neurological changes or death [9]. Contrary to the immediate and rapid clinical effect of primary brain damage caused by a blow to the head, secondary brain injury can occur within minutes or even days after the event. The severity of secondary injury determines mortality in patients recovering from a first traumatic injury. Neuroinflammation plays a role in the pathophysiology of both primary and secondary injuries in TBI [9]. Therefore, the use of inflammatory biomarkers, including CRP, albumin, CAR, and PCT, in predicting mortality is widespread.
C-reactive protein (CRP) is elevated as an acute response to inflammation after tissue injury and TBI. Studies have shown that CRP can be used to determine prognosis and severity after head trauma [10]. Albumin, which is a nutritional status marker, significantly decreases after TBI [11]. CAR, which is an inflammation-based score, has been used for the prognosis and mortality of many diseases by evaluating both inflammation and nutritional status [12]. CAR in TBI was first used by Wang et al. [5], and high levels were found to be associated with poor outcome in TBI. In the same study, CAR was found to be superior to other biomarkers according to logistic regression and ROC curve analysis of CRP, albumin, and CAR [6]. We obtained similar results in our study, where CAR was superior to CRP and albumin in predicting mortality.
PCT, a calcitonin propeptide that is produced by alternative processing of the calcitonin/calcitonin-gene-related-peptide (CGRP) gene transcript, is produced by thyroid C cells, the lungs, and the intestine under physiological conditions [13,14]. Under normal conditions, PCT is found in very low level in serum, but the level increases significantly in cases of bacterial sepsis, multiorgan failure, and systemic inflammation [15]. The increase in serum level of PCT has drawn attention in TBI [8]. While PCT is detected in cerebrospinal fluid after TBI in children, it has been shown that CGRP neuropeptide accumulates in infant rats in a hypoxic-ischemic brain injury model [13,16]. It has also been shown that PCT is released from intestinal neuroendocrine cells into the bloodstream during acute cerebral infarction [17]. In our study, the serum level of PCT was elevated in TBI, and its relationship with mortality was verified. The performance of CAR and PCT in predicting mortality in TBI was similar (AUROC for PCT: 0.646 [95% confidence interval, 0.506–0.785], AUROC for CAR: 0.642 [0.513–0.771]). GCS is considered an important parameter in determining mortality in TBI; however, its performance in predicting mortality is controversial. Studies have shown that new biomarkers are more effective than GCS in predicting mortality [6]. Similar results were obtained in our study. Compared to GCS, the performance of both CAR and PCT biomarkers is superior in predicting mortality.
PCT release increases within three to four hours after induction, reaching the highest serum level at approximately six hours and is known to plateau for 24 hours. In contrast, CRP begins to be synthesized in the liver with inflammation caused by tissue damage, and high serum level is reached after 12–18 hours [18]. Therefore, it takes a longer time to detect disease-related values of CRP in serum relative to PCT. This suggests that PCT can provide more accurate results in the early estimation of mortality. In addition, bleeding after TBI, surgical procedures, and interventional procedures can affect the reliability of CAR by causing changes in CRP and albumin serum levels. Considering the peak times of these markers in serum, serial measurements within the first 24 hours after trauma can determine the most appropriate time and cut-off value for mortality estimation.
Contrary to our study, serial PCT measurements were performed on the first, second, and fifth days for prediction of mortality in TBI patients with or without extracranial injury by Goyal et al. [19], and it was concluded that PCT did not support the prediction of mortality in TBI. When the PCT values of the living and deceased patients were compared, the high PCT levels in deceased patients were statistically significant; however, it was thought that the PCT levels might reveal risk in terms of secondary complications. High PCT level in cases of developing sepsis is an expected result [19]. In our study, a single PCT data point was evaluated in isolated TBI patients. PCT was compared with CAR and GCS to determine its role in mortality prediction.
Our study has potential limitations. First is the retrospective design of the study. Second is the use of a single ICU data point. In addition, only moderate and severe TBI cases were evaluated in our study, making it difficult to interpret the performance of CAR and PCT in predicting mortality in patients with mild TBI. CRP, albumin, and PCT values were obtained from the data within 24 hours after trauma. The results show that CAR and PCT can be used in the earliest hours after trauma to predict mortality. Further prospective study with intermittent measurements is required.
This study showed that PCT and CAR are available biomarkers to predict mortality in TBI. Using the cut-of values determined to predict the course of the disease should provide more effective communication about treatment planning and preparation of the family for this process and their expectations.
▪ This study compared C-reactive protein-albumin ratio (CAR) and procalcitonin (PCT) in predicting intensive care unit (ICU) mortality in traumatic brain injury and shows both are useful markers.
▪ There was a statistically significant difference between PCT and CAR values according to mortality (P=0.041 and P=0.047, respectively).
▪ When the determined cut-off values are used to predict the course of the disease, the CAR and PCT biomarkers provide effective information for treatment planning and for preparation of the family for the treatment process and to manage their outcome expectations.
REFERENCES
1. Traumatic brain injury: time to end the silence. Lancet Neurol. 2010; 9:331.
2. Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974; 2:81–4.
3. Udekwu P, Kromhout-Schiro S, Vaslef S, Baker C, Oller D. Glasgow Coma Scale score, mortality, and functional outcome in head-injured patients. J Trauma. 2004; 56:1084–9.

4. Gao Q, Yuan F, Yang XA, Zhu JW, Song L, Bi LJ, et al. Development and validation of a new score for predicting functional outcome of neurocritically ill patients: the INCNS score. CNS Neurosci Ther. 2020; 26:21–9.

5. Wang R, He M, Ou X, Xie X, Kang Y. CRP albumin ratio is positively associated with poor outcome in patients with traumatic brain injury. Clin Neurol Neurosurg. 2020; 195:106051.

6. Finnie JW. Neuroinflammation: beneficial and detrimental effects after traumatic brain injury. Inflammopharmacology. 2013; 21:309–20.

7. Sakran JV, Michetti CP, Sheridan MJ, Richmond R, Waked T, Aldaghlas T, et al. The utility of procalcitonin in critically ill trauma patients. J Trauma Acute Care Surg. 2012; 73:413–8.

8. Sauerland S, Hensler T, Bouillon B, Rixen D, Raum MR, Andermahr J, et al. Plasma levels of procalcitonin and neopterin in multiple trauma patients with or without brain injury. J Neurotrauma. 2003; 20:953–60.

9. O’Brien WT, Pham L, Symons GF, Monif M, Shultz SR, McDonald SJ. The NLRP3 inflammasome in traumatic brain injury: potential as a biomarker and therapeutic target. J Neuroinflammation. 2020; 17:104.

10. Belavić M, Jančić E, Mišković P, Brozović-Krijan A, Bakota B, Žunić J. Secondary stroke in patients with polytrauma and traumatic brain injury treated in an Intensive Care Unit, Karlovac General Hospital, Croatia. Injury. 2015; 46 Suppl 6:S31–5.

11. Montalcini T, Moraca M, Ferro Y, Romeo S, Serra S, Raso MG, et al. Nutritional parameters predicting pressure ulcers and short-term mortality in patients with minimal conscious state as a result of traumatic and non-traumatic acquired brain injury. J Transl Med. 2015; 13:305.

12. Zhang Y, Zhou GQ, Liu X, Chen L, Li WF, Tang LL, et al. Exploration and validation of C-reactive protein/albumin ratio as a novel inflammation-based prognostic marker in nasopharyngeal carcinoma. J Cancer. 2016; 7:1406–12.

13. Han YY, Carcillo JA, Ruppel RA, Adelson PD, Wisniewski SR, Bell MJ, et al. Cerebrospinal fluid procalcitonin and severe traumatic brain injury in children. Pediatr Crit Care Med. 2002; 3:39–44.

14. Zhang Y, Liu G, Wang Y, Su Y, Leak RK, Cao G. Procalcitonin as a biomarker for malignant cerebral edema in massive cerebral infarction. Sci Rep. 2018; 8:993.

15. Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta. 2002; 323:17–29.

16. Dragunow M, Sirimanne E, Lawlor PA, Williams C, Gluckman P. Accumulation of calcitonin-gene related peptide-like immunoreactivity after hypoxic-ischaemic brain injury in the infant rat. Brain Res Mol Brain Res. 1992; 14:267–72.

17. Li YM, Liu XY. Molecular mechanisms underlying application of serum procalcitonin and stool miR-637 in prognosis of acute ischemic stroke. Am J Transl Res. 2016; 8:4242–9.
18. Gregoriano C, Heilmann E, Molitor A, Schuetz P. Role of procalcitonin use in the management of sepsis. J Thorac Dis. 2020; 12(Suppl 1):S5–15.

19. Goyal K, Tomar GS, Sengar K, Singh GP, Aggarwal R, Soni KD, et al. Prognostic value of serially estimated serum procalcitonin levels in traumatic brain injury patients with or without extra cranial injury on early in-hospital mortality: a longitudinal observational study. Neurocrit Care. 2021; 34:182–92.

Figure 1.
Flowchart displaying selective and exclusive process of patients with severe traumatic brain injury (TBI) in the current study. ICU: intensive care unit.
Figure 2.
Receiver operating characteristic (ROC) curves displaying predictive value of procalcitonin (PCT), C-reactive protein to albumin ratio (CAR) and Glasgow Coma Scale (GCS) score for traumatic brain injury.
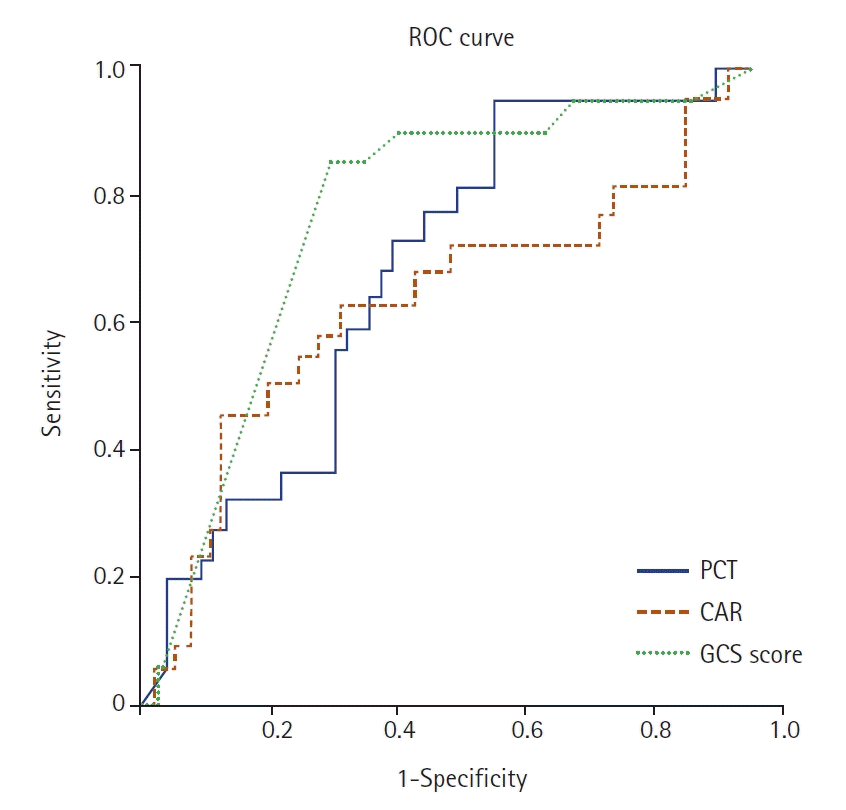
Table 1.
ROC curves for the PCT, CAR, GCS, CRP, and albumin compared to ICU mortality
Table 2.
Patient demographic characteristics and results between death and survival
Values are presented as mean±standard deviation or number (%).
DM: Diabetes Mellitus; HT: Hypertension; ACS: Acute Coronary Syndrome; ICU: intensive care unit; APACHE: Acute Physiology and Chronic Health Evaluation; GCS: Glasgow Coma Scale; PCT: procalcitonin; CAR: C-reactive protein to albumin ratio; CRP: C-reactive protein.
Table 3.
Diagnostic scanning for the PCT and CAR
| Diagnostic scanning test | Value |
|---|---|
| PCT | |
| Cut-off | 1.166 |
| Sensitivity (%) | 65.2 |
| Specificity (%) | 66.1 |
| PPV | 38.5 |
| NPV | 81.4 |
| CAR | |
| Cut-off | 0.32 |
| Sensitivity (%) | 61 |
| Specificity (%) | 60.09 |
| PPV | 37.8 |
| NPV | 80 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download