1. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020; 91:157–60.
2. Bajema KL, Dahl RM, Evener SL, Prill MM, Rodriguez-Barradas MC, Marconi VC. Comparative effectiveness and antibody responses to moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans: five veterans affairs medical centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70:1700–5.
3. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384:403–16.

4. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383:2603–15.

6. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506.

7. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020; 46:846–8.

8. Zhang L, Hou J, Ma FZ, Li J, Xue S, Xu ZG. The common risk factors for progression and mortality in COVID-19 patients: a meta-analysis. Arch Virol. 2021; 166:2071–87.

9. Booth A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One. 2021; 16:e0247461.

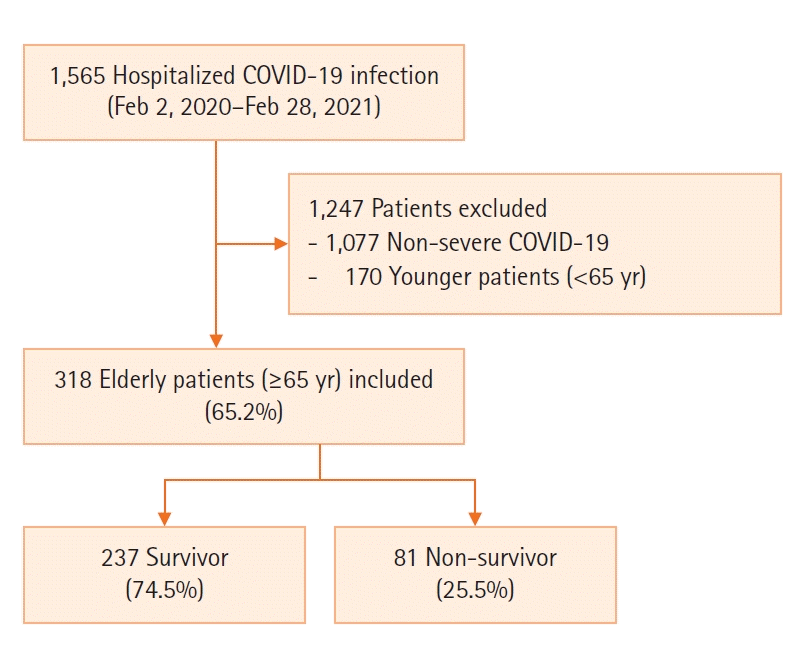
10. Guo T, Shen Q, Guo W, He W, Li J, Zhang Y, et al. Clinical characteristics of elderly patients with COVID-19 in Hunan Province, China: a multicenter, retrospective study. Gerontology. 2020; 66:467–75.

11. Singhal S, Kumar P, Singh S, Saha S, Dey AB. Clinical features and outcomes of COVID-19 in older adults: a systematic review and meta-analysis. BMC Geriatr. 2021; 21:321.

12. Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020; 80:e14–e18.

13. Pepe M, Maroun-Eid C, Romero R, Arroyo-Espliguero R, Fernàndez-Rozas I, Aparisi A, et al. Clinical presentation, therapeutic approach, and outcome of young patients admitted for COVID-19, with respect to the elderly counterpart. Clin Exp Med. 2021; 21:249–68.

14. Owen RK, Conroy SP, Taub N, Jones W, Bryden D, Pareek M, et al. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: a retrospective observational study using electronic health records. Age Ageing. 2021; 50:307–16.

15. Aw D, Woodrow L, Ogliari G, Harwood R. Association of frailty with mortality in older inpatients with Covid-19: a cohort study. Age Ageing. 2020; 49:915–22.

16. Pranata R, Henrina J, Lim MA, Lawrensia S, Yonas E, Vania R, et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch Gerontol Geriatr. 2021; 93:104324.

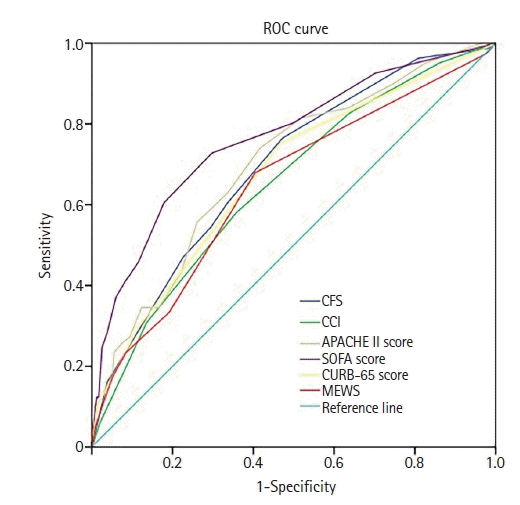
17. Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017; 317:290–300.

18. Yang Z, Hu Q, Huang F, Xiong S, Sun Y. The prognostic value of the SOFA score in patients with COVID-19: a retrospective, observational study. Medicine (Baltimore). 2021; 100:e26900.

19. Pulok MH, Theou O, van der Valk AM, Rockwood K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing. 2020; 49:1071–9.

20. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020; 383:1757–66.

21. Vrillon A, Hourregue C, Azuar J, Grosset L, Boutelier A, Tan S, et al. COVID-19 in older adults: a series of 76 patients aged 85 years and older with COVID-19. J Am Geriatr Soc. 2020; 68:2735–43.

22. Herrmann ML, Hahn JM, Walter-Frank B, Bollinger DM, Schmauder K, Schnauder G, et al. COVID-19 in persons aged 70+ in an early affected German district: Risk factors, mortality and post-COVID care needs: a retrospective observational study of hospitalized and non-hospitalized patients. PLoS One. 2021; 16:e0253154.
23. Knopp P, Miles A, Webb TE, Mcloughlin BC, Mannan I, Raja N, et al. Presenting features of COVID-19 in older people: relationships with frailty, inflammation and mortality. Eur Geriatr Med. 2020; 11:1089–94.

24. Lee JY, Kim HA, Huh K, Hyun M, Rhee JY, Jang S, et al. Risk factors for mortality and respiratory support in elderly patients hospitalized with COVID-19 in Korea. J Korean Med Sci. 2020; 35:e223.

25. Leung C. Risk factors for predicting mortality in elderly patients with COVID-19: a review of clinical data in China. Mech Ageing Dev. 2020; 188:111255.

26. Gao S, Jiang F, Jin W, Shi Y, Yang L, Xia Y, et al. Risk factors influencing the prognosis of elderly patients infected with COVID-19: a clinical retrospective study in Wuhan, China. Aging (Albany NY). 2020; 12:12504–16.

27. Li P, Chen L, Liu Z, Pan J, Zhou D, Wang H, et al. Clinical features and short-term outcomes of elderly patients with COVID-19. Int J Infect Dis. 2020; 97:245–50.

28. Tuty Kuswardhani RA, Henrina J, Pranata R, Anthonius Lim M, Lawrensia S, Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020; 14:2103–9.

29. Joynt GM, Leung AK, Ho CM, So D, Shum HP, Chow FL, et al. Admission triage tool for adult intensive care unit admission in Hong Kong during the COVID-19 outbreak. Hong Kong Med J. 2022; 28:64–72.

30. Sprung CL, Joynt GM, Christian MD, Truog RD, Rello J, Nates JL. Adult ICU triage during the coronavirus disease 2019 pandemic: who will live and who will die? Recommendations to improve survival. Crit Care Med. 2020; 48:1196–202.

31. Aziz S, Arabi YM, Alhazzani W, Evans L, Citerio G, Fischkoff K, et al. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. 2020; 46:1303–25.

32. Jung C, Flaatten H, Fjølner J, Bruno RR, Wernly B, Artigas A, et al. The impact of frailty on survival in elderly intensive care patients with COVID-19: the COVIP study. Crit Care. 2021; 25:149.
33. Aliberti MJ, Szlejf C, Avelino-Silva VI, Suemoto CK, Apolinario D, Dias MB, et al. COVID-19 is not over and age is not enough: using frailty for prognostication in hospitalized patients. J Am Geriatr Soc. 2021; 69:1116–27.

34. Berenguer J, Borobia AM, Ryan P, Rodríguez-Baño J, Bellón JM, Jarrín I, et al. Development and validation of a prediction model for 30-day mortality in hospitalised patients with COVID-19: the COVID-19 SEIMC score. Thorax. 2021; 76:920–9.

35. Alves VP, Casemiro FG, Araujo BG, Lima MA, Oliveira RS, Fernandes FT, et al. Factors associated with mortality among elderly people in the COVID-19 pandemic (SARS-CoV-2): a systematic review and meta-analysis. Int J Environ Res Public Health. 2021; 18:8008.

36. Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021; 21:855.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download