Abstract
Background
A subdural hematoma (SDH) following a traumatic brain injury (TBI) in children can lead to unexpected death or disability. The nomogram is a clinical prediction tool used by physicians to provide prognosis advice to parents for making decisions regarding treatment. In the present study, a nomogram for predicting outcomes was developed and validated. In addition, the predictors associated with outcomes in children with traumatic SDH were determined.
Methods
In this retrospective study, 103 children with SDH after TBI were evaluated. According to the King’s Outcome Scale for Childhood Head Injury classification, the functional outcomes were assessed at hospital discharge and categorized into favorable and unfavorable. The predictors associated with the unfavorable outcomes were analyzed using binary logistic regression. Subsequently, a two-dimensional nomogram was developed for presentation of the predictive model.
Results
The predictive model with the lowest level of Akaike information criterion consisted of hypotension (odds ratio [OR], 9.4; 95% confidence interval [CI], 2.0–42.9), Glasgow coma scale scores of 3–8 (OR, 8.2; 95% CI, 1.7–38.9), fixed pupil in one eye (OR, 4.8; 95% CI, 2.6–8.8), and fixed pupils in both eyes (OR, 3.5; 95% CI, 1.6–7.1). A midline shift ≥5 mm (OR, 1.1; 95% CI, 0.62–10.73) and co-existing intraventricular hemorrhage (OR, 6.5; 95% CI, 0.003–26.1) were also included.
Traumatic brain injury (TBI) is a cause of mortality and functional disability in children, particularly in low- and medium-income countries [1-3]. Following TBI, a subdural hematoma (SDH) is a common intracranial injury that requires intensive care. Binder et al. [4] reported a prognosis of traumatic SDH in 47 children with a mortality rate and vegetative state of 11% and 2%, respectively. In addition, Beni-Adani et al. [5] reported 20% mortality in children with SDH in their study cohort. Prognostic factors for traumatic SDH studied in which literature review include age, Glasgow coma scale (GCS) score, and pupillary abnormalities. The maximal thickness of SDH, presence of subarachnoid hemorrhage (SAH), presence of parenchymal lesions, degree of midline shift, and basal cistern effacement are additional factors [6-9]. Based on literature review, evidence is lacking for predictors associated with prognosis in children with traumatic SDH, which is problematic due to unexpected death, disability, and low health-related quality of life.
In an era of disruptive technology, various clinical prediction tools have been developed and used for prognosis in various conditions including TBI [10], oncology [11], and surgical complications [12]. A nomogram is a prediction instrument used for outcome prediction, especially TBI. Tunthanathip et al. [13] proposed a clinical nomogram for predicting traumatic intracranial injury in children. The tool had an area under the receiver operating characteristic curve (AUC) of 0.71. Cui et al. [10] developed a nomogram for predicting prognosis in TBI patients after decompressive craniectomy and showed an AUC of 0.83. Furthermore, Parmontree et al. [14] proposed a nomogram for prediction of early posttraumatic seizure, with an AUC of 0.77. Predictors associated with prognosis in pediatric SDH have been reported in a limited number of studies. Therefore, in the present study, a nomogram was developed and validated for predicting such outcomes. In addition, the predictors associated with outcomes in children with traumatic SDH were determined.
This clinical investigation was approved by the Institutional Review Board Ethics Committee of Prince of Songkla University (REC.64-250-10-1). Informed consent was not required from patients due to the retrospective study design. In addition, patient identification numbers were encoded before analysis.
The patients in this study were treated at a trauma center in southern Thailand. The subjects were <15 years of age when enrolled from January 1, 2009, to December 31, 2020. However, patients who died before arrival, died within the first 24 hours following injury, or had unavailable cranial computed tomography were excluded. The clinical information and imaging finding of the children were collected with a structured form, including age, sex, signs, symptoms, GCS score, and mechanism of injury. The severity of TBI was classified based on GCS score as follows: mild TBI (GCS scores 13–15), moderate TBI (GCS scores 9–12), and severe TBI (GCS scores 3–8) [2,3]. The co-existing intracranial injuries including epidural hematoma, contusion, intraventricular hemorrhage (IVH), and SAH were evaluated for information from the cranial region. In addition, the degree of midline shift and obliteration of the basal cistern were estimated by two neurosurgeons.
Using the King’s Outcome Scale for Childhood Head Injury (KOSCHI) classification, the functional outcome in the present study was assessed at hospital discharge and 6-month follow-up [15]. In detail, KOSCHI was categorized into favorable (good recovery and moderate disability) and unfavorable outcomes (severe disability, vegetative state, and death) for binary classifier proposes [3].
Clinical characteristics and imaging findings were calculated from descriptive statistics and reported as percentages for categorical data and mean±standard deviation (SD) for continuous variables. Predictors were estimated using binary logistic regression analysis for predictive model development. The candidate predictors were identified at a P<0.10. Thus, the candidate variables were entered into multivariable analysis for the final model based on the backward elimination method. Any P-values <0.05 were considered statistically significant, and the Akaike information criterion (AIC) with clinical relevance was used for model selection [16,17].
Assessment of the predictive model’s performance consisted of two domains, calibration and discrimination. For calibration, the Hosmer-Lemeshow goodness-of-fit (GOF) test and the calibration plot were performed, and a GOF test P-value ≥0.05 indicated good calibration of the model [18]. Discrimination of the model refers to the predictability of a model to differentiate between binary classifiers. The concordance statistic index (c-index) or AUC was measured to indicate discriminatory ability [18,19]. Consequently, internal validation was performed to observe the overfitting problems of the model. Resampling techniques were used for both 1,000 bootstrapping and five cross-validations in the present study. The results of internal validation were reported as the optimism-corrected c-index for both techniques [19-21]. Finally, the predictive model exhibition was utilized following model performances and internal validation as a two-dimensional nomogram. The statistical analysis was performed using R version 4.4.0 software (R Foundation, Vienna, Austria).
Demographic information is listed in Table 1. The mean age of the patients was 141.8 months (SD, 48.0 months); 20.4% of the present cohort were children <5 years of age. More than two-thirds (64.1%) were male, and motorcycle accident was the most common mechanism of injury. In addition, road traffic injury was the major cause of TBI, comprising 84.5% of all cases. Common findings from the physical examination were scalp injury, loss of consciousness, and amnesia, and 9.7% of cases experienced hypotension episodes at the emergency department. Furthermore, early posttraumatic seizure occurred in 5.8% of the present study cohort. Lower extremity injury was the most common associated injury in the present cohort, and isolated TBI was observed in 75.7% of cases. In addition, hypotension was not significantly associated with multiple organ injuries (chi-square test, P=0.74)
Imaging findings after cranial computed tomography are shown in Table 2. All cases of SDH were acute, and hematomas were commonly located in the frontal area, tentorium cerebelli/falx cerebri, and temporal region. The common co-existing injuries were cerebral contusion (37.9%), calvaria skull fracture (33.0%), and SAH (30.1%). Furthermore, obliteration of the basal cistern and midline shift ≥5 mm were observed in 10.7% and 6.8% of cases, respectively. In the present study, surgical treatment was performed in 14.6% of subjects. Decompressive craniectomy was performed in 7.8% of subjects and intracranial monitoring in 1.9% of all cases. One child underwent burr hole due to bilateral chronic SDH. The mean length of hospital stay in the present cohort was 10.4 days (SD, 7.4 days), and the mortality rate at hospital discharge was 8.7%. Based on the KOSCHI at hospital discharge, good recovery, moderate disability, and severe disability were found in 76.7%, 5.8%, and 7.8%, respectively, of patients. When the 6-month follow-up KOSCHI was administered, good recovery, moderate disability, severe disability, and death were found in 77.7%, 4.9%, 8.7%, and 8.7% of subjects, respectively. Therefore, 17.5% of the present study cohort had an unfavorable outcome at hospital discharge, as shown in Table 3.
Clinical variables were analyzed using univariate logistic regression as shown in Table 4. The following 12 variables were candidate predictors: age group, hypotension, amnesia, GCS score, pupillary light reflex, IVH, SAH, thickness of epidural hematoma, lateralization of SDH, obliteration of basal cistern, midline shift, and treatment. Therefore, candidate predictors were analyzed using multivariable analysis. Following the backward elimination procedure, the final model with the lowest AIC consisted of hypotension (odds ratio [OR], 9.4; 95% confidence interval [CI], 2.0–42.9), GCS score of 3–8 (OR, 8.2; 95% CI, 1.7–38.9), a fixed pupil in one eye (OR, 4.8; 95% CI, 2.6–8.8), and fixed pupils in both eyes (OR, 3.5; 95% CI, 1.6–7.1). In addition, a midline shift ≥ 5 mm (OR, 1.1; 95% CI, 0.62–10.73) and co-existing IVH (OR, 6.5; 95% CI, 0.003–26.1) were also included in the final model, as shown in Figure 1.
The domain of the model discrimination was estimated using the Hosmer-Lemeshow GOF test with a P-value of 0.99 and a c-index of 0.971, indicating good calibration. The domain of the model calibration was evaluated based on the calibration plot, which was the logistic calibration (intercept, −3.790; slope, 7.286), as shown in Figure 2. For internal validation, the overfitting performance of the model was evaluated using 1,000 bootstrapping and five cross-validations. The c-index values of bootstrapping and cross-validation were 0.971 and 0.971, respectively.
The c-index of the predictive model was outstanding without overfitting. Therefore, the final predictive model was finalized into a nomogram for predicting the unfavorable outcomes of new patients at the 6-month follow-up, as shown in Figure 3.
SDH following TBI leads to mortality and disability in patients, especially children. The mortality rate of SDH in children was reported to range from 11% to 20% in prior studies. Tunthanathip et al. [3] studied 948 children with TBI and found SDH to be significantly associated with unfavorable outcomes. However, studies in which SDH is specifically investigated in a young population are lacking, resulting in increased burden of healthcare cost and poor health-related quality of life. In the present study, a predictive tool to identify children with increased risk of an unfavorable outcome was developed and validated, providing physicians with information for advising parents and making treatment decisions in clinical practice.
Using multivariable analysis, the predictive model consisted of the following five predictors: hypotension, GCS score, pupillary light reflex, midline shift, and IVH. Hypotension was the strongest predictor, in agreement with previous studies [3,22,23]. In general, systolic blood pressure has been considered significantly associated with mortality in TBI. In prior studies, blood loss from multiple organ injuries was shown to cause hypotension [13,14]. However, the majority of patients in the present study experienced isolated TBI, and hypotension was not significantly associated with multiple organ injuries. Episodes of hypotension were directly associated with low cerebral perfusion pressure and unfavorable outcomes [24]. In addition, a pupillary light reflex indicated reduced function of the brainstem due to alteration of cerebral perfusion or downward cerebral herniation [25].
In the present study, low GCS score was significantly associated with poor outcomes, in concordance with previous studies. Dent et al. and Petridis et al. reported that low GCS score affected the outcomes in SDH patients [6,7]. Specifically, children with low initial GCS score suffered severe brain tissue damage, lowering the likelihood of brain recovery. Based on the lowest AIC, the final predictive model had two non-significant predictors in multivariable analysis; however, they were reported as prognostic factors with clinical relevance in the literature. In prior studies, the presence of IVH and high midline shift significantly increased the risk of unfavorable outcomes after treatment [26,27]. However, the limited prevalence of those factors needs to be collected in the future to increase the power of testing the hypothesis. A nomogram from a predictive model was previously developed and estimated and had an excellent c-index level [28]. However, the overfitting performance is a common optimism problem that should be evaluated using internal validation [19-21]. Using five cross-validations and bootstrapping methods, the optimism-corrected c-indices did not decrease the value, which confirmed no overfitting performance in the nomogram [20,21,29].
The present study had several limitations. This was a single-center study with a retrospective design, which might have led to some bias. However, the authors attempted to adjust the confounders using multivariable analysis. In addition, a multicenter study should be conducted in the future for external validation of the nomogram. To the best of our knowledge, this is the first study in which a clinical nomogram for predicting outcomes in children with traumatic SDH was proposed, and the predictability performance was compared between the nomogram and other prediction tools such as machine learning [30,31].
SDH in pediatric TBI leads to mortality and disability and is associated with poor outcomes that apparently are the consequence of associated injuries and clinical status. The predictability level of the nomogram in the present study was excellent, and external validation should be performed to confirm the predictability of the tool.
▪ Subdural hematoma (SDH) in pediatric traumatic brain injury is associated with mortality and disability resulting in disease burden in developing nations.
▪ The prognostic factors for traumatic SDH were hypotension, Glasgow coma scale score 3–8, pupillary light reflex, midline shift ≥5 mm, and co-existing intraventricular hemorrhage.
▪ The nomogram is a prediction tool used to help physicians make treatment decisions.
REFERENCES
1. Peden M, Oyegbite K, Ozanne-Smith J, Hyder AA, Branche C, Rahman AK, et al. World report on child injury prevention. Geneva: World Health Organization; 2008.
2. Taweesomboonyat C, Kaewborisutsakul A, Tunthanathip T, Saeheng S, Oearsakul T. Necessity of in-hospital neurological observation for mild traumatic brain injury patients with negative computed tomography brain scans. J Health Sci Med Res. 2020; 38:267–74.

3. Tunthanathip T, Phuenpathom N. Impact of road traffic injury to pediatric traumatic brain injury in Southern Thailand. J Neurosci Rural Pract. 2017; 8:601–8.

4. Binder H, Tiefenboeck TM, Majdan M, Komjati M, Schuster R, Hajdu S, et al. Management and outcome of traumatic subdural hematoma in 47 infants and children from a single center. Wien Klin Wochenschr. 2020; 132:499–505.

5. Beni-Adani L, Flores I, Spektor S, Umansky F, Constantini S. Epidural hematoma in infants: a different entity? J Trauma. 1999; 46:306–11.
6. Dent DL, Croce MA, Menke PG, Young BH, Hinson MS, Kudsk KA, et al. Prognostic factors after acute subdural hematoma. J Trauma. 1995; 39:36–42.

7. Petridis AK, Dörner L, Doukas A, Eifrig S, Barth H, Mehdorn M. Acute subdural hematoma in the elderly: clinical and CT factors influencing the surgical treatment decision. Cent Eur Neurosurg. 2009; 70:73–8.

8. Moussa WM, Khedr WM, Elwany AH. Prognostic significance of hematoma thickness to midline shift ratio in patients with acute intracranial subdural hematoma: a retrospective study. Neurosurg Rev. 2018; 41:483–8.

9. Hiraizumi S, Shiomi N, Echigo T, Oka H, Hino A, Baba M, et al. Factors associated with poor outcomes in patients with mild or moderate acute subdural hematomas. Neurol Med Chir (Tokyo). 2020; 60:402–10.

10. Cui W, Ge S, Shi Y, Wu X, Luo J, Lui H, et al. Death after discharge: prognostic model of 1-year mortality in traumatic brain injury patients undergoing decompressive craniectomy. Chin Neurosurg J. 2021; 7:24.

11. Tunthanathip T, Ratanalert S, Sae-Heng S, Oearsakul T, Sakaruncchai I, Kaewborisutsakul A, et al. Prognostic factors and nomogram predicting survival in diffuse astrocytoma. J Neurosci Rural Pract. 2020; 11:135–43.

12. Tang TY, Zong Y, Shen YN, Guo CX, Zhang XZ, Zou XW, et al. Predicting surgical site infections using a novel nomogram in patients with hepatocelluar carcinoma undergoing hepatectomy. World J Clin Cases. 2019; 7:2176–88.

13. Tunthanathip T, Duangsuwan J, Wattanakitrungroj N, Tongman S, Phuenpathom N. Comparison of intracranial injury predictability between machine learning algorithms and the nomogram in pediatric traumatic brain injury. Neurosurg Focus. 2021; 51:E7.

14. Parmontree P, Tunthanathip T, Doungngern T, Rojpitbulstit M, Kulviwat W, Ratanalert S. Predictive risk factors for early seizures in traumatic brain injury. J Neurosci Rural Pract. 2019; 10:582–7.

15. Calvert S, Miller HE, Curran A, Hameed B, McCarter R, Edwards RJ, et al. The King's outcome scale for childhood head injury and injury severity and outcome measures in children with traumatic brain injury. Dev Med Child Neurol. 2008; 50:426–31.

16. Harrison SM, Wei MY, Lamerato LE, Petrie JG, Toth Martin E. Multimorbidity is associated with uptake of influenza vaccination. Vaccine. 2018; 36:3635–40.

17. Vahedi M, Mahmoodi M, Mohammad K, Ossareh S, Zeraati H. What is the best parametric survival models for analyzing hemodialysis data? Glob J Health Sci. 2016; 8:56161.

18. Hosmer DW, Lemeshow S. A goodness of fit test for the multiple logistic regression model. Commun Stat Theory Methods. 1980; 9:1043–69.
19. Han K, Song K, Choi BW. How to develop, validate, and compare clinical prediction models involving radiological parameters: study design and statistical methods. Korean J Radiol. 2016; 17:339–50.

20. Austin PC, Steyerberg EW. Interpreting the concordance statistic of a logistic regression model: relation to the variance and odds ratio of a continuous explanatory variable. BMC Med Res Methodol. 2012; 12:82.

21. Tunthanathip T. Development of nomogram for neurosurgery. In: Tunthanathip T, editor. Translational medicine in neuro-surgery. Bangkok: Sahamit Pattana Printing; 2022. p. 111-31.
22. Berry C, Ley EJ, Bukur M, Malinoski D, Margulies DR, Mirocha J, et al. Redefining hypotension in traumatic brain injury. Injury. 2012; 43:1833–7.

23. Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017; 80:6–15.

24. Hutchison JS, Frndova H, Lo TY, Guerguerian AM; Hypothermia Pediatric Head Injury Trial Investigators; Canadian Critical Care Trials Group. Impact of hypotension and low cerebral perfusion pressure on outcomes in children treated with hypothermia therapy following severe traumatic brain injury: a post hoc analysis of the Hypothermia Pediatric Head Injury Trial. Dev Neurosci. 2010; 32:406–12.

25. Vathanalaoha K, Oearsakul T, Tunthanathip T. Predictive factors of survival and 6-month favorable outcome of very severe head trauma patients; a historical cohort study. Emerg (Tehran). 2017; 5:e24.
26. Atzema C, Mower WR, Hoffman JR, Holmes JF, Killian AJ, Wolfson AB, et al. Prevalence and prognosis of traumatic intraventricular hemorrhage in patients with blunt head trauma. J Trauma. 2006; 60:1010–7.

27. LeRoux PD, Haglund MM, Newell DW, Grady MS, Winn HR. Intraventricular hemorrhage in blunt head trauma: an analysis of 43 cases. Neurosurgery. 1992; 31:678–84.
28. Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010; 5:1315–6.

29. Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001; 54:774–81.
30. Tunthanathip T, Sae-Heng S, Oearsakul T, Sakarunchai I, Kaewborisutsakul A, Taweesomboonyat C. Machine learning applications for the prediction of surgical site infection in neurological operations. Neurosurg Focus. 2019; 47:E7.

31. Tunthanathip T. Machine learning for neurosurgery. In: Tunthanathip T, editor. Translational medicine in neurosurgery. Bangkok: Sahamit Pattana Printing; 2022. p. 135-72.
Figure 1.
Forest plot of the adjusted odds ratio (OR) of predictors. CI: confidence interval; GCS: Glasgow coma scale; IVH: intraventricular hemorrhage.
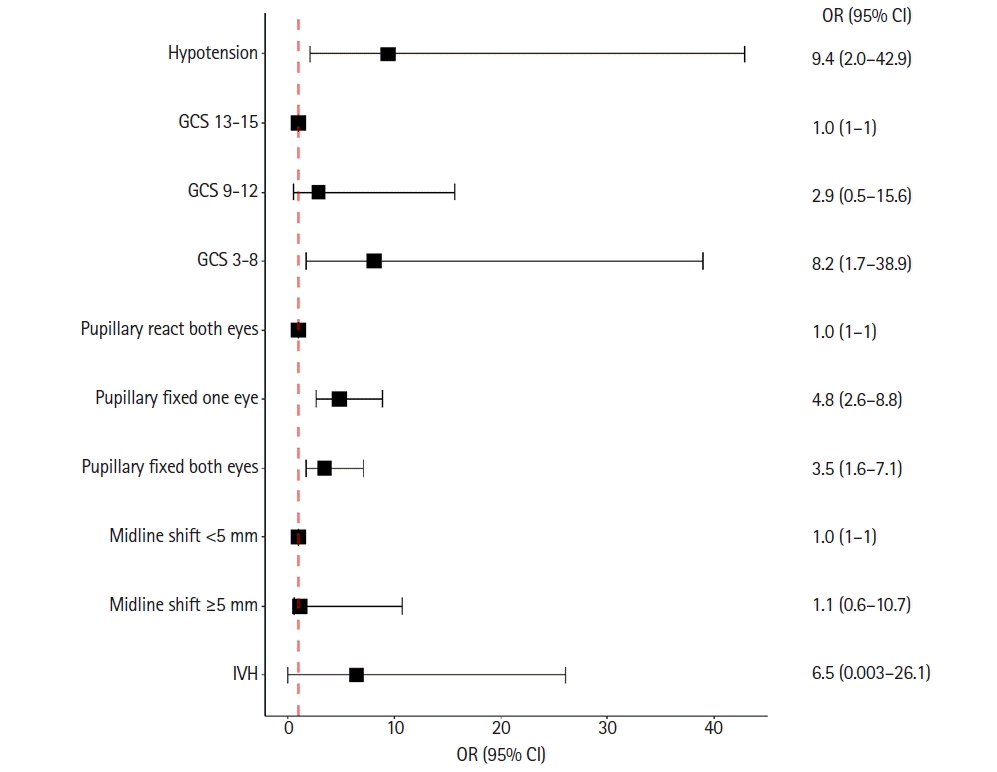
Figure 2.
Calibration plot of the predictive model. Dxy: somers' dxy rank correlation; C (ROC): concordance statistic (area under the receiver operating characteristic curve).
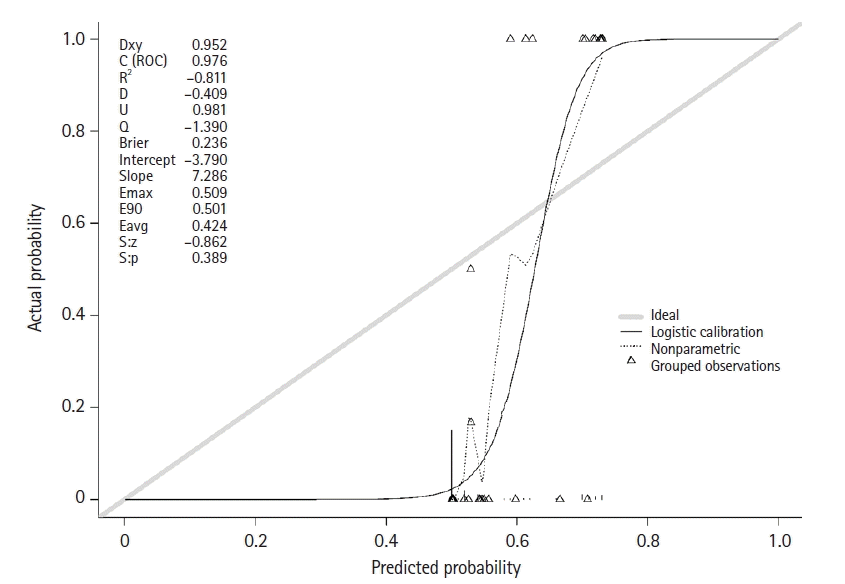
Figure 3.
Nomogram predicting unfavorable outcomes in children with traumatic subdural hematoma. GCS: Glasgow coma scale; IVH: intraventricular hemorrhage; MLS: midline shift.
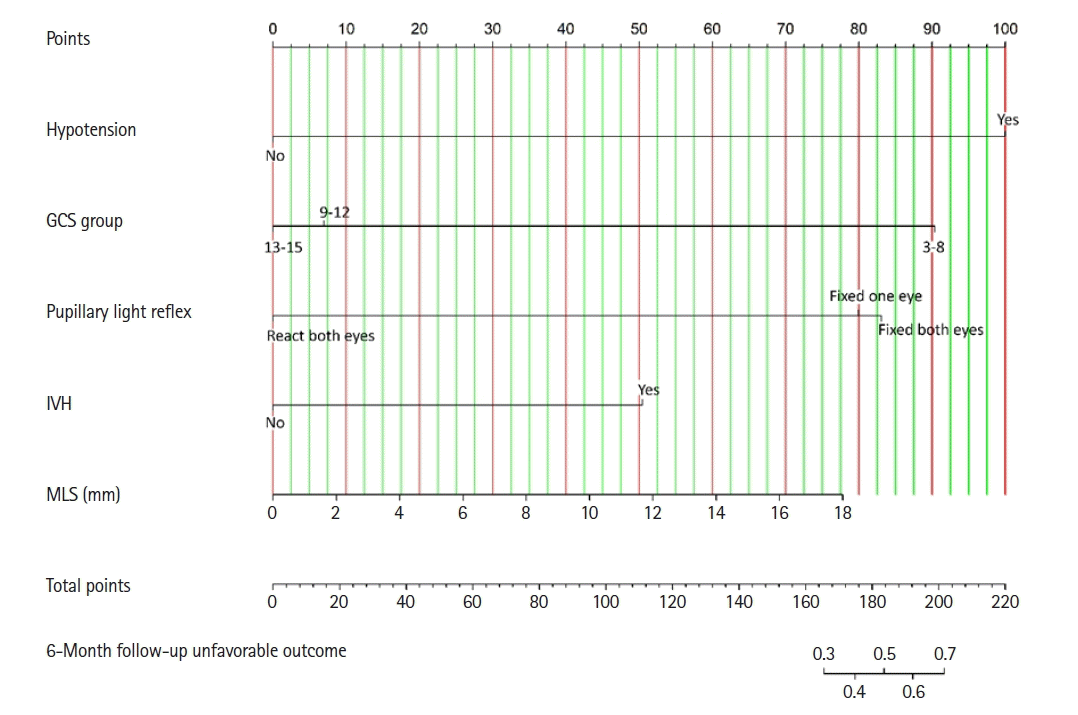
Table 1.
Characteristics of children with traumatic subdural hematoma (n=103)
Table 2.
Imaging finding of children with traumatic SDH (n=103)
Table 3.
Treatment and outcome of children with traumatic subdural hematoma (n=103)
Table 4.
Binary logistic regression analysis for unfavorable outcome




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download