Abstract
Background
Early intensive care unit (ICU) protocolized rehabilitative programs have been described previously, yet with differing starting time points and mostly on mechanically ventilated patients. We extended the concept to all admitted ICU patients and investigate the efficacy of early mobilization in improving mobility of the critically ill, address issues surrounding the timing and intensity of an early rehabilitative program.
Methods
Prospective cohorts of patients admitted consecutively before-and-after (control, n=92; intervention, n=90) the introduction of an early mobilization program in a single center, general hospital ICU. Improvement in mobility as assessed by ICU mobility score, on ICU admission and upon ICU discharge, was measured as a primary outcome.
Results
Those receiving early mobilization in the intensive care unit had higher ICU mobility score (2.63; 95% confidence interval, 0.65–4.61; P<0.001) upon discharge from the intensive care, with earlier out of bed mobilization on day 5 compared to the control group of day 21 (P<0.001). No differences were found in terms of mortality, intensive care hospitalization and subsequent hospitalization duration after discharge from ICU.
An admission to the intensive care unit for many patients, despite advances in diagnostics and therapeutics and general reductions in mortality, remains an event where many are left with significant post intensive care morbidities [1]. Most patients remain bedridden for prolonged periods during their intensive care stay and have significant reductions in mobility, as seen in the worsening of frailty [1-4]. Early initiation of rehabilitation was previously described but with mixed results, where in general there was an observed reduction in length of stay for mechanically ventilated patients [5-7]. To our knowledge, most rehabilitative interventions begin at different time points after admission to the intensive care unit, ranging from the first day of admission to the first day of liberation from ventilation [3,8], while some specifying the commencement of mobilization after 5 days of intensive care hospitalization [9]. Thus, the definition of “early mobilization” remains unclear, while differences between early mobilization and standard care are often poorly described [10]. This also brings to question of what and when constitutes early mobilization and is an appropriate level of mobilization during the course of acute illnesses.
In particular, mechanically ventilated patients suffer most with increased use of muscle relaxants and sedatives [2]. Clinically this results in a worsening of frailty, mobility and many are at increased risk of delirium. A previous program using early initiation of early whole-body physiotherapy resulted in more ventilator free days, shortened delirium and return to baseline functional states. This use of an early rehabilitative program also included reducing or daily cessation of sedation and its titration against a recognized sedation scoring system, and training in functional independence [5]. Some authors also describe the importance of a team composed of all staff within the intensive care unit, in addition to physiotherapists, due to the scarcity of physiotherapist resources [6,11].
However, many patients who are not mechanically ventilated may also benefit from early initiation of a rehabilitative program. Many similarly suffer from reduced mobility and may have multi-organ impairments. Further, there is a trend towards reduced use of mechanical ventilation for various respiratory conditions [12-14]. Hence, it is equally important to explore the use of an early rehabilitative program on non-mechanically ventilated individuals.
Aims of the investigation are as follows. (1) Investigate the efficacy of early mobilization in all intensive care patients; (2) Explore the optimal timing for key steps of the mobilization program; (3) Identify specific predictors of outcomes with the mobilization exercise performed.
This early rehabilitative program is a before-and-after study, with an initial observation period in April to October of 2017 as control group, and subsequently an intervention group from April to October 2020 in a single center, 15-bed intensive care unit and with an approximately 650-bed district general hospital with general medical, surgical and orthopedic capacities. In both periods, patients underwent the same selection criteria for initiation of physiotherapy within the intensive care unit. In our hospital locality, despite the coronavirus disease 2019 (COVID-19) pandemic, our hospital or intensive care capacity was not overwhelmed during the period of intervention in question, allowing fairly suitable comparison of data between the two time periods.
Patients are screened within 24 hours of intensive care unit admission. In addition to mechanically ventilated patients, we include all consecutively admitted medical, surgical and orthopedic patients except whom early physiotherapy is not possible, namely those on prone ventilation, active hypertensive emergency, bradycardia on active pharmacological therapy or awaiting pacemaker insertion, uncontrolled intracranial pressure, active seizures, unstable or suspected unstable spinal injuries, rapidly developing neuromuscular diseases and active uncontrolled hemorrhage. All patients were adults (>18 years old). We consider patients completing an initial program once they have 3 physiotherapy sessions.
COVID-19 patients were excluded from our study as we were unable to provide dedicated equipment to all COVID-19 patients and hence, may not have received all aspects of our early physiotherapy program. Further, COVID-19 was not a known disease in the control group with improving therapies throughout 2020 and direct comparisons are thus invalid.
This intervention program consists of a program with a range of mobilization exercises performed, with sedation titrated against the Richmond Agitation Sedation Score (RASS) of 0 to –2 for mechanically ventilated patients [5]. Patients admitted to the ICU are evaluated within 24 hours by a physiotherapist who is experienced in critical care physiotherapy for their ability to begin mobilization. Physiotherapy is performed at least once a day, with daily cessation or reduction in sedation as appropriate, and early mobilization is delivered by physiotherapists and/or trained nursing staff in mobilization, led by a senior physiotherapist responsible for oversight of the early mobilization program at the unit. For unresponsive patients, passive limb mobilization is performed for at least 30 minutes daily. Otherwise, patients receive mobilization on the bed. Out-of-bed mobilization, such as transfer training, sitting out of bed with assistance and assisted cycling or upper limb exercises in supported sitting, is initiated once no exclusion criteria is met for such mobilization. Subsequently, patients progress stepwise to dangling and sitting at edge-of-bed when limb power reaches at least grade 3 in the Medical Research Council Muscle (MRC) scale. Patients not reaching this continue passive or assisted active mobilization out-of-bed. Once limb power of MRC grading reaches 4 with satisfactory truncal control, other out-of-bed mobilization such as standing at bedside with aids is attempted with subsequent gradual reduction in standing assistance and an attempt on marching on spot. This prepares for the final achievement stage of walking, which is defined as the ability to walk for at least 5 meters with or without aids (Figure 1, Supplementary Material 1).
Outcome measurements are defined a priori. Primary outcome measurement is the improvement in mobility score, where mobility score is assessed according to the ICU Mobility Scale, with the pre-score recorded in the first mobilization session and the post-score at the final assessment upon discharge from the intensive care unit by the attending physiotherapist. Prior studies show excellent inter-rater agreement between physiotherapists [15]. Secondary outcome measurements include mortality, hospitalization days, and the time to achieving a mobility score. Safety details are reviewed, noting the cause of termination against a predefined set of criteria for cessation of exercise (Table 1). The subjects’ clinical notes were also reviewed for any potential complications during physiotherapy such as dislodgement of catheters, patient fall or injury, which were routinely documented according to the local protocols of the intensive care unit.
For analysis of length of stay and mobility score differences, multivariate linear regression analysis is performed, which includes main effect and two-way interaction analyses. Mortality is analyzed using Cox proportional hazard model, whereas analysis of the length of time and selected mobilization stages is done using Mantel-Cox test. Otherwise, categorical data is analyzed using chi-square test and continuous data utilizes Wilcoxon signed-rank test. Analysis is performed using R ver. 3.6.0. (RStudio), whilst graphing output is performed on Prism 9 (GraphPad, San Diego, CA, USA).
Reporting in this work adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Ethical review was sought and approved from the local Institutional Review Board of Prince of Wales Hospital (No. 2021.396) with waiver for individual consent, and this work complies with the Declaration of Helsinki. Individual consent was not applicable due to the before-and-after, consecutive nature of the program.
The characteristics of patients on admission to the intensive care unit are described in Table 2. With the exception of the number of ventilated patients, namely a reduction in mechanically ventilated patients seen in the early mobilization cohort, all other variables including the Acute Physiology and Chronic Health Evaluation (APACHE) IV scores and the predicted mortality for all patients were essentially similar.
In the analysis of length of hospital stay, no differences were found in the intensive care hospitalization nor after discharge from the intensive care (Figure 2A and B). The 30- and 60-day mortality analysis using a Cox proportional hazard model shown no difference in mortality (Figure 2C). Ninety days mortality analysis also showed no difference but is not shown due to the small number of participants and wide overlapping confidence intervals.
Subgroup analysis with three specific subgroups of patients, namely mechanically ventilated patients, postoperative patients, and severely ill patients, did not yield statistically significant results. This was expected due to the relatively small number of patients in each subgroup.
Despite similar length of intensive care hospitalization, we observed an improvement in mobility scoring of 2.63 (95% confidence interval, 0.65–4.61; P<0.001) (Figure 2D) within intensive care hospitalization in the group receiving early mobilization, a substantial improvement from the usual mobility score of 1–2 (mean, 1.8; standard deviation, 1.8) in the control cohort.
In the early mobilization group, patients were able to achieve higher mobility scores earlier on in the course of their intensive care unit stay. While there is no difference in limb mobilization achievements in the control group of day 2 and early mobilization group of day 1 (P=0.39), mobilization out of bed is the first stage showing differences in outcomes, with the control group mobilising out of bed on day 21 compared to day 5 (P<0.001) of the early mobilization group. While only one patient achieved walking in the control group at day 11 (median undefined), 44.4% were able to do so in the early mobilization group at a median of day 14 (P<0.001) (Figure 2E).
Early mobilization was well tolerated in the majority of patients, with the distribution of a predefined criterion for stopping mobilization similar to the control group (Table 1). Due to low numbers of failure in early mobilization, in analysis with grouped patient factors, cardiovascular-respiratory and other reasons for cessation of exercise, comparison with the control group showed no differences in terms of the frequency of cessation of exercise (P=0.25, n=1,325; chi-square test). We did not note any major safety events leading to direct patient harm.
An extension towards all admitted patients yielded similar intensive care hospitalization days but did not show a reduction in post intensive care hospitalization days. However, patients who were involved in an early rehabilitative program had substantially better mobility scores compared to the control group. Importantly, this mean discharge mobility score indicates an ability for basic self-function in the subsequent general ward stay [16,17]. In keeping with our expectations, no mortality difference was observed between the cohorts. Importantly, surgical and orthopedic patients can also benefit from increased mobilization as the effect is not limited to mechanically ventilated patients.
Classically, most intensive care patients are minimally mobilized and the majority of physiotherapy performed evolves around chest physiotherapy [3,4,18]. Earlier mobilization had been proposed by various authors with good results but without clear consensus on the “earliness.” To our knowledge, no literature currently defines the timeframe in the performance of certain targets as early, while most studies opt to define early mobilization merely as the commencement of mobilization within a certain timeframe or suggest optimal times for beginning mobilization [6-8,10,19].
We show that limb mobilization can generally be performed by day 1 of intensive care unit admission, whereas mobilization out of bed can mostly be achieved by day 5 of intensive care admission. Limb mobilization itself is frequently performed in all patients even prior to the establishment of an early physiotherapy program and does not have any value in predicting subsequent mobility. Further works teasing out the effect of each mobilization step, including its omission, will prove interesting. Although initial results do question the value of passive limb mobilization, where its effect alone is absent in terms of length of stay and unclear in prevention of contractures in neurological diseases [6,20], it may provide theoretical advantage in preparation for the next mobility phase [21].
Importantly, sitting out of bed itself is the major achievement offered by such a program. This posture is associated with improved oxygenation and respiratory mechanics [22,23]. Further, this posture requires substantial truncal tone and lays the foundation for further standing and walking exercises [24,25]. This is important as out of bed mobilization is most strongly associated with reduced weakness and function, including at after discharge from hospital [17].
We also show that day 14 is an achievable target for walking, and in addition a larger proportion of patients were also able to walk in the early mobilization group in comparison to the control group. Walking, including in-patient walking in general wards, has been associated with preservation of functional independence and reduced frailty, which are themselves valuable outcomes [26,27]. Our data reveals certain early attainable targets and can serve as a reference for further works in this topic, although we caution against setting targets based on a rigid timeframe without correlation to the clinical context of the patient and employ rigorous clinical appraisal of limb strength prior to extending targets of mobilization.
Despite this, we found no effect as described by interaction terms regarding the dose effect of physiotherapy, which is defined by the surrogate marker of the number of physiotherapy sessions given. This again is in coherence with previous analysis on the dose of physiotherapy [6,26], although we specifically excluded in analysis of patients who did not receive a minimum of 3 physiotherapy sessions. Hence, the attainment of an out-of-bed target for most patients, with progress tailored to their tolerance, is perhaps a key determining factor of success.
One adverse event was detected, namely blood staining over the insertion site of a radial arterial catheter, immediately after mobilization. A review of the event showed that no issues with catheter integrity and its usage was continued for the subsequent 24 hours. The most common reasons for early termination are tachycardia and patient refusal. Increases in heart rate beyond 20 bpm is likely due to mobilization exercises causing physical exertion. Overall, the results of our study agree with previous work in this field, indicating the feasibility and relative safety of early mobilization [28-30], as we did not find any differences in the reasons for cessation of physiotherapy session in both groups. Interestingly, contrary to previous works, we did not find nurse or physician concern to be a factor for early termination [11]. Possible explanations can be the involvement of trained nurses in mobilization as part of the physiotherapy delivery team.
The assessment of mechanically ventilated patients in our dataset is limited by the differences in the proportion of ventilated individuals despite an overall similar severity of illness presented. This is most likely a random effect but may also represent changes due to the general trends in intensive care, or could be an improvement due to the initiation of an early mobilization program [12-14]. We are unable to determine the magnitude of the effect with these important caveats, and hence the differences seen in ventilated patients must be interpreted with caution, despite seemingly reaffirming prior studies where number of ventilated days are reduced in an early mobilization group [5,6,31]. Furthermore, as limited by the number of subjects in this study, we are unable to determine the subgroup of patients, such as according to disease severity, mechanical ventilation or postoperative status, benefit most from our early mobilization program. Although the outcomes after discharge to general ward and their subsequent discharge home are of great interest to assess the aftereffects of a critical care episode, this was not possible as it was not measured.
Early mobilization during intensive care can be offered for all intensive care patients and improves patient mobility scores upon discharge from the intensive care unit. Early mobilization for all patients however is not associated with differences in terms of mortality, intensive care hospitalization and the length of stay after intensive care hospitalization. Further, we show that very early and moderately intense mobilization can be done with a substantial number of participants able to mobilize out of bed within 5 days, and a substantial proportion of patients can walk by day 14 of initial intensive care unit admission. Prospective studies done in future may elucidate the long-term impact and to clarify the effects of each segment of the program.
▪ Protocolized early mobilization in the intensive care is effective in improving patient mobility scores.
▪ Median time taken for patients to mobilize out-of-bed is 5 days after intensive care unit admission.
▪ Tolerability of early mobilization in critically ill patients is high and without major adverse events.
ACKNOWLEDGMENTS
The authors would like to express our appreciation and gratitude to the dedicated nurses, physiotherapists and healthcare assistants in their invaluable assistance and encouragement of critically ill patients.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2021.01564.
REFERENCES
1. Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013; 310:1591–600.

2. Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014; 370:1626–35.

3. Nydahl P, Ruhl AP, Bartoszek G, Dubb R, Filipovic S, Flohr HJ, et al. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. 2014; 42:1178–86.
4. Sibilla A, Nydahl P, Greco N, Mungo G, Ott N, Unger I, et al. Mobilization of mechanically ventilated patients in Switzerland. J Intensive Care Med. 2020; 35:55–62.

5. Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009; 373:1874–82.

6. Waldauf P, Jiroutková K, Krajčová A, Puthucheary Z, Duška F. Effects of rehabilitation interventions on clinical outcomes in critically ill patients: systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2020; 48:1055–65.
7. Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017; 43:171–83.

8. Harrold ME, Salisbury LG, Webb SA, Allison GT; Australia and Scotland ICU Physiotherapy Collaboration. Early mobilisation in intensive care units in Australia and Scotland: a prospective, observational cohort study examining mobilisation practises and barriers. Crit Care. 2015; 19:336.

9. Denehy L, Skinner EH, Edbrooke L, Haines K, Warrillow S, Hawthorne G, et al. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care. 2013; 17:R156.

10. Menges D, Seiler B, Tomonaga Y, Schwenkglenks M, Puhan MA, Yebyo HG. Systematic early versus late mobilization or standard early mobilization in mechanically ventilated adult ICU patients: systematic review and meta-analysis. Crit Care. 2021; 25:16.

11. Hodgson CL, Capell E, Tipping CJ. Early mobilization of patients in intensive care: organization, communication and safety factors that influence translation into clinical practice. Crit Care. 2018; 22:77.

12. Schnell D, Timsit JF, Darmon M, Vesin A, Goldgran-Toledano D, Dumenil AS, et al. Noninvasive mechanical ventilation in acute respiratory failure: trends in use and outcomes. Intensive Care Med. 2014; 40:582–91.

13. de Miguel-Diez J, Jiménez-García R, Hernández-Barrera V, Puente-Maestu L, Girón-Matute WI, de Miguel-Yanes JM, et al. Trends in the use and outcomes of mechanical ventilation among patients hospitalized with acute exacerbations of COPD in Spain, 2001 to 2015. J Clin Med. 2019; 8:1621.

14. Demoule A, Chevret S, Carlucci A, Kouatchet A, Jaber S, Meziani F, et al. Changing use of noninvasive ventilation in critically ill patients: trends over 15 years in francophone countries. Intensive Care Med. 2016; 42:82–92.

15. Hodgson C, Needham D, Haines K, Bailey M, Ward A, Harrold M, et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung. 2014; 43:19–24.

16. Parry SM, Denehy L, Beach LJ, Berney S, Williamson HC, Granger CL. Functional outcomes in ICU: what should we be using?: an observational study. Crit Care. 2015; 19:127.
17. Paton M, Lane R, Paul E, Cuthburtson GA, Hodgson CL. Mobilization during critical illness: a higher level of mobilization improves health status at 6 months, a secondary analysis of a prospective cohort study. Crit Care Med. 2021; 49:e860–9.

18. Lottering M, Van Aswegen H. Physiotherapy practice in South African intensive care units. South Afr J Crit Care. 2016; 32:11–6.

19. Ding N, Zhang Z, Zhang C, Yao L, Yang L, Jiang B, et al. What is the optimum time for initiation of early mobilization in mechanically ventilated patients?: a network meta-analysis. PLoS One. 2019; 14:e0223151.

20. Prabhu RK, Swaminathan N, Harvey LA. Passive movements for the treatment and prevention of contractures. Cochrane Database Syst Rev. 2013; (12):CD009331.

21. Kwah LK, Herbert RD, Harvey LA, Diong J, Clarke JL, Martin JH, et al. Passive mechanical properties of gastrocnemius muscles of people with ankle contracture after stroke. Arch Phys Med Rehabil. 2012; 93:1185–90.

22. Lemyze M, Mallat J, Duhamel A, Pepy F, Gasan G, Barrailler S, et al. Effects of sitting position and applied positive end-expiratory pressure on respiratory mechanics of critically ill obese patients receiving mechanical ventilation. Crit Care Med. 2013; 41:2592–9.

23. Hickmann CE, Montecinos-Munoz NR, Castanares-Zapatero D, Arriagada-Garrido RS, Jeria-Blanco U, Gizzatullin T, et al. Acute effects of sitting out of bed and exercise on lung aeration and oxygenation in critically ill subjects. Respir Care. 2021; 66:253–62.

24. Saeys W, Vereeck L, Truijen S, Lafosse C, Wuyts FP, Heyning PV. Randomized controlled trial of truncal exercises early after stroke to improve balance and mobility. Neurorehabil Neural Repair. 2012; 26:231–8.

25. Lanzetta D, Cattaneo D, Pellegatta D, Cardini R. Trunk control in unstable sitting posture during functional activities in healthy subjects and patients with multiple sclerosis. Arch Phys Med Rehabil. 2004; 85:279–83.

26. Killey B, Watt E. The effect of extra walking on the mobility, independence and exercise self-efficacy of elderly hospital in-patients: a pilot study. Contemp Nurse. 2006; 22:120–33.

27. Billot M, Calvani R, Urtamo A, Sánchez-Sánchez JL, Ciccolari-Micaldi C, Chang M, et al. Preserving mobility in older adults with physical frailty and sarcopenia: opportunities, challenges, and recommendations for physical activity interventions. Clin Interv Aging. 2020; 15:1675–90.
28. Hodgson CL, Stiller K, Needham DM, Tipping CJ, Harrold M, Baldwin CE, et al. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care. 2014; 18:658.

29. Brummel NE, Girard TD, Ely EW, Pandharipande PP, Morandi A, Hughes CG, et al. Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the Activity and Cognitive Therapy in ICU (ACT-ICU) trial. Intensive Care Med. 2014; 40:370–9.

Figure 1.
Flowchart showing an overview of the study protocol and description of each incremental steps of the early mobilization program offered to the interventional group. Patients are recruited within 24 hours of admission to the intensive care unit. Assessment for mobilization after initial recruitment is performed daily according to the listed steps. MRC: Medical Research Council.
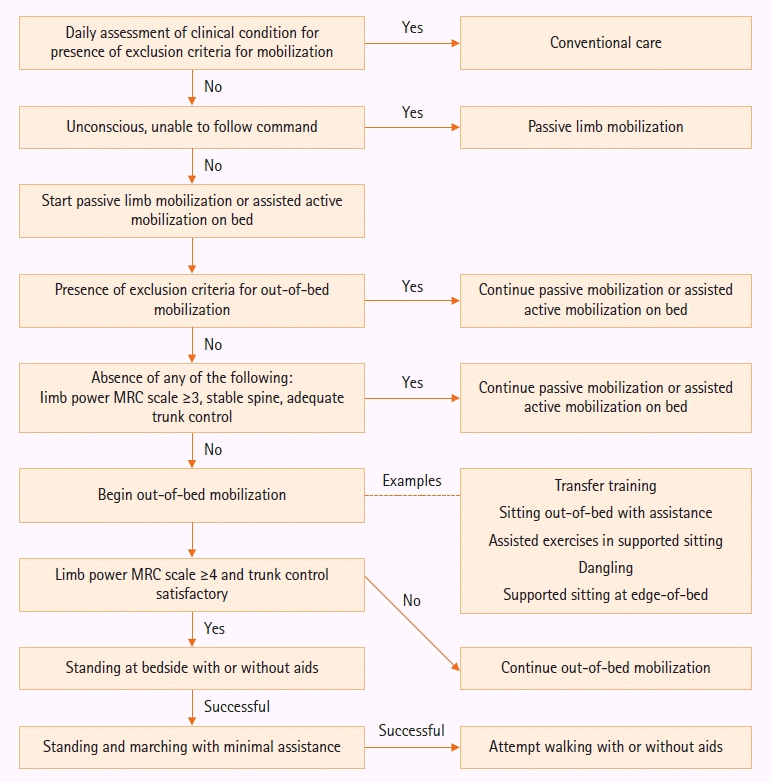
Figure 2.
Summary of results. (A) The length of hospitalization in the intensive care unit (ICU) of the control group (n=92; median, 6; interquartile range [IQR], 4–9) and the early mobilization group (n=90; median, 6; IQR, 4–10) show no differences (P=0.89; 95% confidence interval [CI], –6.86 to 6.00; R2=0.95). (B) The length of hospitalization in general ward after discharge from the intensive care. The control group (n=92; median, 11; IQR, 4.25–24) and the early mobilization group (n=90; median, 11; IQR, 4–19) show no differences (P=0.72; 95% CI, –134 to 94; R2=0.31). (C) Survival analysis of the control group and the early mobilization group shows no significance at 30 and 60 days after ICU admission (P=0.27). Shaded areas within the dotted lines represent the 95% CI. (D) ICU Mobility Scale on admission of the control cohort (mean, 0.8; standard deviation [SD], 0.4; blue) and early mobilization cohort (mean, 1.2; SD, 1.5); and upon discharge in control group (mean, 1.8, SD, 1.8) and early mobilization group (mean, 4.9; SD, 2.9). There is a significant improvement in the mobilization scores (2.63, P<0.0001; 95% CI, 0.65–4.61). (E) Time taken to achieve the selected mobilization targets. All data, censored and events, are marked.
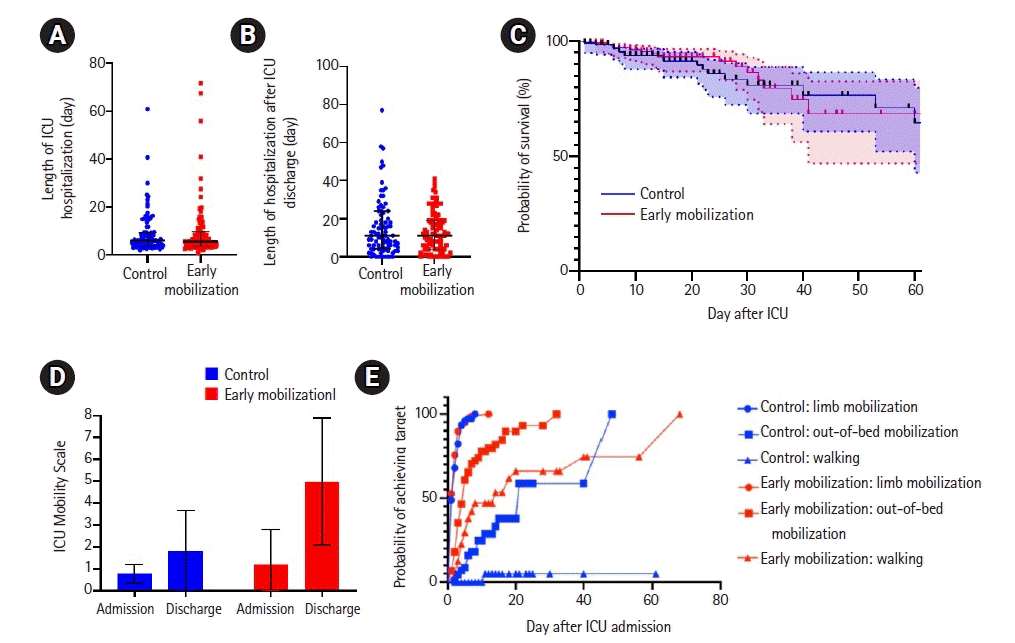
Table 2.
Characteristics of the control group and the group receiving early mobilization on their admission to the intensive care unit
Table 1.
Predefined criteria for cessation of mobilization
Values are presented as number (%). The reason, number and percentage of premature termination of early mobilization are shown. All physiotherapy sessions in the control and the early mobilization groups are reviewed with no missing data. In the control group, 20 patients had early termination of physiotherapy with 31 total sessions terminated. The total sessions terminated was 39 while the number of patients with terminated sessions was 24 (n=90) in the early mobilization group.
BP: blood pressure; RR: respiratory rate; SpO2: oxygen saturation; ECG: electrocardiogram; ST: ST segment.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download