Abstract
Background
Methods
Results
ACKNOWLEDGMENTS
REFERENCES
Figure 1.
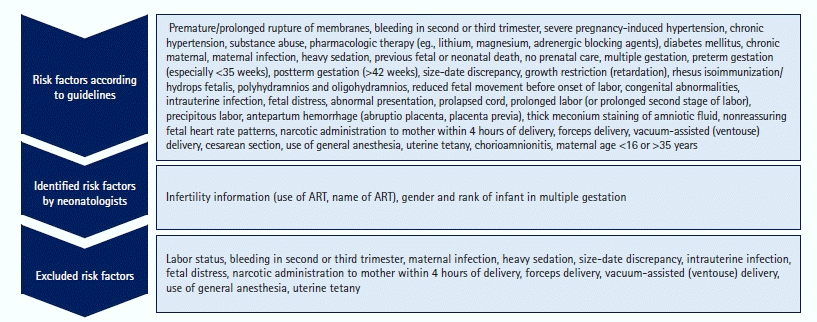
Figure 2.
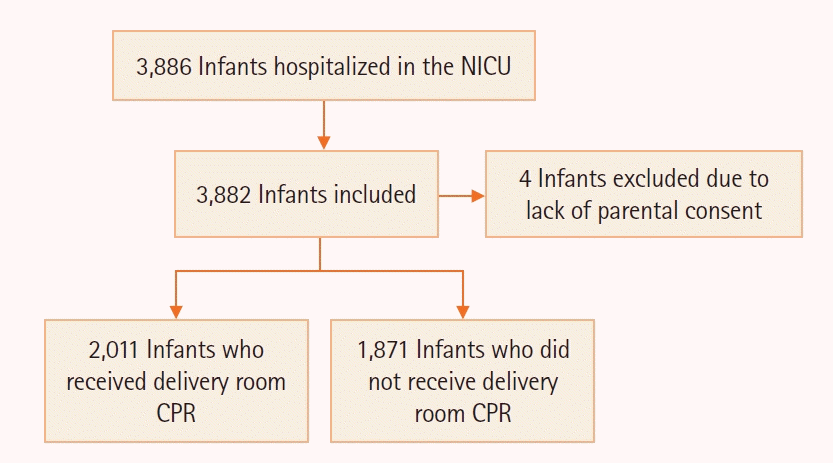
Figure 3.
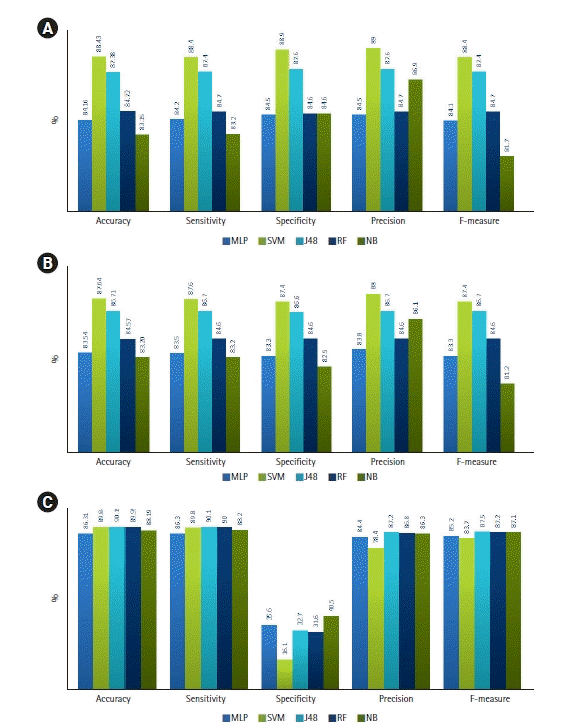
Figure 4.
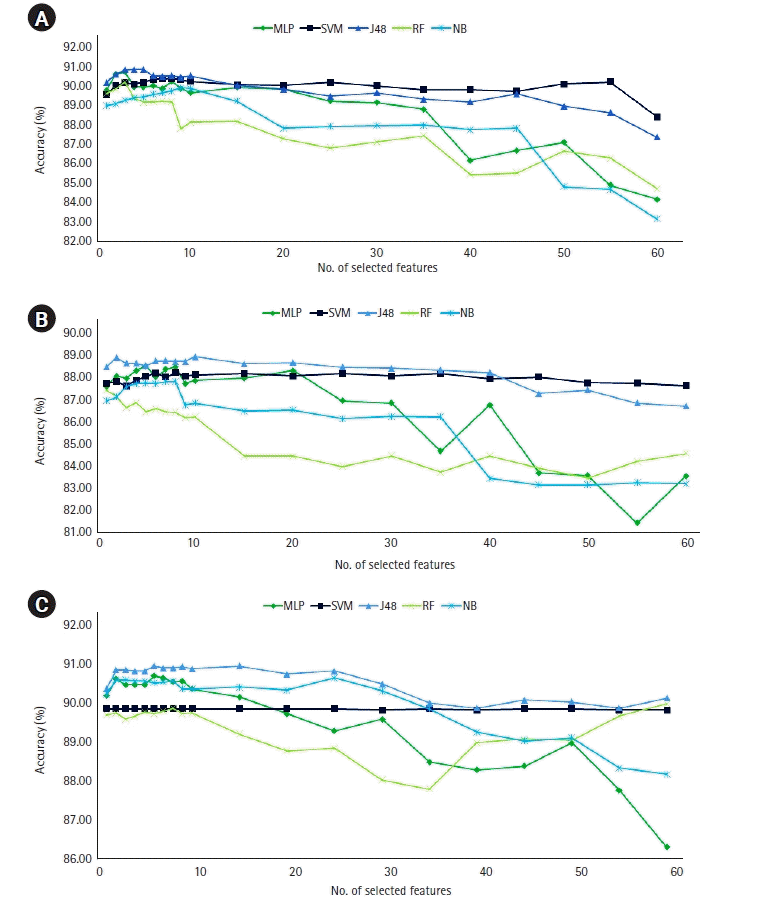
Figure 5.
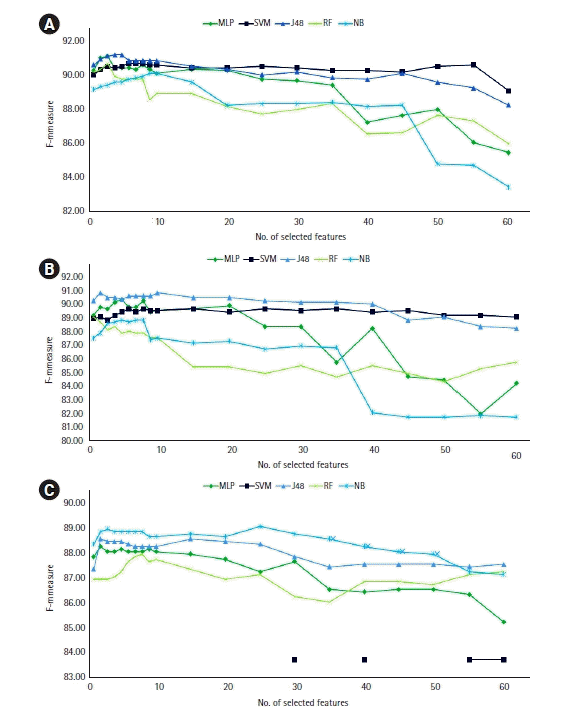
Table 1.
Table 2.
| Variable | Total (n=3,882) | CPR (n=2,011) | Non-CPR (n=1,871) | P-value (CPR vs. non-CPR group) |
|---|---|---|---|---|
| Gestational risk factor | ||||
| Prenatal care | 3,426 (88.25) | 1837 | 1589 | <0.001 |
| Chorioamnionitis | 71 (1.83) | 37 | 34 | 0.958 |
| Steroid administration | 933 (24.03) | 647 | 286 | <0.001 |
| Magnesium sulfate administration | 333 (8.58) | 238 | 95 | <0.001 |
| Maternal risk factor | ||||
| Hypertension | 184 (4.74) | 113 | 71 | 0.008 |
| Gestational hypertension | 654 (16.85) | 379 | 275 | 0.001 |
| Diabetes | 105 (2.71) | 53 | 52 | 0.783 |
| Gestational diabetes | 600 (15.46) | 317 | 283 | 0.583 |
| Mother addiction | 63 (1.62) | 22 | 41 | 0.007 |
| Mother HIV | 28 (0.72) | 14 | 14 | 0.848 |
| Cardiac disease | 304 (7.83) | 142 | 162 | 0.064 |
| Blood disease | 187 (4.82) | 98 | 89 | 0.866 |
| Kidney disease | 63 (1.62) | 33 | 30 | 0.926 |
| Thyroid disorders | 0.274 | |||
| Hyperthyroidism | 15 (0.39) | 8 | 7 | |
| Hypothyroidism | 694 (17.88) | 344 | 350 | |
| Thyroidectomy | 2 (0.05) | 0 | 2 | |
| Respiratory disease | 28 (0.72) | 15 | 13 | 0.851 |
| Mental disease | 21 (0.54) | 11 | 10 | 0.958 |
| Infectious disease | 16 (0.41) | 8 | 8 | 0.885 |
| Brain diseases | 62 (1.6) | 37 | 25 | 0.211 |
| Cancer disease | 33 (0.85) | 19 | 14 | 0.505 |
| Skin disease | 7 (0.18) | 3 | 4 | 0.635 |
| Liver disease | 63 (1.62) | 32 | 31 | 0.872 |
| Autoimmune disease | 64 (1.65) | 32 | 32 | 0.771 |
| Uterus disease | 41 (1.06) | 23 | 18 | 0.580 |
| Digestive disease | 34 (0.88) | 15 | 19 | 0.368 |
| Eye disease | 4 (0.10) | 2 | 2 | 0.942 |
| Other chronic disease | 12 (0.31) | 9 | 3 | 0.107 |
| Pre-eclampsia | 0.192 | |||
| Eclampsia | 8 (0.21) | 4 | 4 | |
| Preeclampsia | 198 (5.10) | 115 | 83 | |
| Abortion history | 17 (0.44) | 9 | 8 | 0.925 |
| Intrauterine fetal death Infertility | 10 (0.26) | 3 | 7 | 0.167 |
| Female infertility | 214 (5.51) | 146 | 68 | <0.001 |
| ART | 144 (3.71) | 94 | 50 | 0.001 |
| Drug | 26 (0.67) | 23 | 3 | <0.001 |
| IUI | 18 (0.46) | 9 | 9 | |
| IVF | 100 (2.58) | 62 | 38 | |
| Accreta status | ||||
| Decollement/placenta abruption | 41 (1.06) | 28 | 13 | 0.034 |
| Vasa previa | 1 (0.03) | 1 | 0 | 0.335 |
| Previa | 113 (2.91) | 62 | 51 | 0.508 |
| Placenta accreta | 163 (4.2) | 94 | 69 | 0.126 |
| Fetal data | ||||
| Number of infants | <0.001 | |||
| 1 | 3,407 (87.76) | 1678 | 1729 | |
| 2 | 419 (10.79) | 293 | 126 | |
| 3 | 55 (1.42) | 39 | 16 | |
| 4 | 1 (0.03) | 1 | 0 | |
| Sex | 0.396 | |||
| Female | 1,730 (44.57) | 881 | 849 | |
| Male | 2,146 (55.28) | 1128 | 1018 | |
| Ambiguous genitalia | 6 (0.15) | 2 | 4 | |
| Rank of infant | <0.001 | |||
| 1 | 3,628 (93.46) | 1838 | 1790 | |
| 2 | 235 (6.05) | 160 | 75 | |
| 3 | 19 (0.49) | 13 | 6 | |
| IUGR | 223 (5.75) | 134 | 89 | 0.011 |
| Tumor | 14 (0.36) | 8 | 6 | 0.689 |
| Genetic problems/anomaly | 18 (0.46) | 13 | 5 | 0.082 |
| Macrosomia | 19 (0.49) | 3 | 16 | 0.002 |
| Cardiac problem | 31 (0.8) | 16 | 15 | 0.983 |
| Surgery (defect of the abdominal) | 54a (1.39) | 24 | 30 | 0.276 |
| Blood problem | 4 (0.10) | 2 | 2 | 0.942 |
| Pulmonary problem | 12 (0.31) | 9 | 3 | 0.107 |
| Brain problem | 25 (0.64) | 13 | 12 | 0.984 |
| Fetal hydrops | 12 (0.31) | 10 | 2 | 0.029 |
| Other problem (fetus) | 6 (0.15) | 3 | 3 | 0.930 |
| Delivery risk factor | ||||
| Delivery type | <0.001 | |||
| Cesarean | 3,617 (93.17) | 1923 | 1694 | |
| Vaginal | 265 (6.83) | 88 | 177 | |
| PRoM | 549 (14.14) | 304 | 245 | 0.071 |
| Presentation | 0.073 | |||
| Breech | 106 (2.73) | 42 | 64 | |
| Transverse | 6 (0.15) | 2 | 4 | |
| Hand | 1 (0.03) | 1 | 0 | |
| Normal | 3,769 (97.09) | 1966 | 1803 | |
| Cord | 0.240 | |||
| Absent Doppler | 27 (0.69) | 18 | 9 | |
| Cord prolapse | 4 (0.10) | 3 | 1 | |
| Reverse | 1 (0.03) | 1 | 0 | |
| No | 3,850 (99.18) | 1989 | 1861 | |
| Thick meconium | 24 (0.62) | 16 | 8 | 0.144 |
| Amniotic fluid | 0.041 | |||
| Oligohydramnios | 43 (1.11) | 18 | 25 | |
| Polyhydramnios | 26 (0.67) | 8 | 18 | |
| Normal | 3,813 (98.22) | 1985 | 1828 | |
| Fetal heart condition | 0.395 | |||
| Arrhythmia | 1 (0.03) | 0 | 1 | |
| BPP | 2 (0.05) | 1 | 1 | |
| Bradycardia | 6 (0.15) | 5 | 1 | |
| Tachycardia | 10 (0.26) | 5 | 5 | |
| Decreased FHR | 269 (6.93) | 148 | 121 | |
| Fetal distress | 8 (0.21) | 6 | 2 | |
| Sinusoidal | 1 (0.03) | 1 | 0 | |
| PVC | 1 (0.03) | 1 | 0 | |
| No | 3,584 (92.31) | 1844 | 1740 | |
| Continuous risk factor | ||||
| Maternal age (yr) | 30.89±5.9 | 30.85±3.81 | 30.94±3.68 | 0.474 |
| Gestational age (day) | 247.15±25.17 | 237.19 ±26.35 | 257.85 ±18.38 | <0.001 |
| PRoM (hr) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | <0.001 |
Values are presented as number (%), mean±standard deviation (range), or median (interquartile range).
CPR: cardiopulmonary resuscitation; HIV: human immunodeficiency virus; ART: assisted reproductive technique; IUI: intrauterine insemination; IVF: in vitro fertilization; IUGR: intrauterine growth restriction; PRoM: prelabor rupture of membranes; BPP: biophysical profile; FHR: fetal heart rate; PVC: premature ventricular contraction.
Table 3.
CPR: cardiopulmonary resuscitation; CFS: correlation-based feature selection; SVM: support vector machine; NB: Naïve Bayesian; ART: assisted reproductive techniques; FHR: fetal heart rate; GA: gestational age; HIV: human immunodeficiency virus; IUFD: intrauterine fetal death; IUGR: intrauterine growth restriction; PRoM: prelabor rupture of membranes.
Table 4.
| ML method |
General CPR |
Basic CPR |
Advanced CPR |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Accuracy | F-measure | Number of selected features | Accuracy | F-measure | Number of selected features | Accuracy | F-measure | Number of selected features | |
| MLP | 90.76 | 90.8 | 3 | 88.51 | 88.5 | 5 | 90.71 | 88.2 | 6, 2a |
| J48 | 90.89 | 90.9 | 4 | 88.92 | 88.9 | 10 | 90.97 | 88.5 | 6, 2a |
| RF | 90.24 | 90.3 | 3 | 87.43 | 87.4 | 1 | 89.76 | 87.7 | 10 |
| SVM | 90.42 | 90.3 | 8 | 88.23 | 87.9 | 8 | 89.86 | 83.7 | 1, 30a |
| NB | 89.93 | 89.6 | 9 | 87.82 | 87.2 | 7 | 90.61 | 88.9 | 3 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download