1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. 2019; Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 157:107843. DOI:
10.1016/j.diabres.2019.107843. PMID:
31518657. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85075731827&origin=inward.

3. Dall TM, Yang W, Gillespie K, Mocarski M, Byrne E, Cintina I, Beronja K, Semilla AP, Iacobucci W, Hogan PF. 2019; The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 42:1661–1668. DOI:
10.2337/dc18-1226. PMID:
30940641. PMCID:
PMC6702607. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071497405&origin=inward.

5. Seferović PM, Polovina M, Bauersachs J, Arad M, Ben Gal T, Lund LH, Felix SB, Arbustini E, Caforio ALP, Farmakis D, Filippatos GS, Gialafos E, Kanjuh V, Krljanac G, Limongelli G, Linhart A, Lyon AR, Maksimović R, Miličić D, Milinković I, et al. 2019; Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 21:553–576. DOI:
10.1002/ejhf.1461. PMID:
30989768. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064571312&origin=inward.

6. Tromp J, Lim SL, Tay WT, Teng TK, Chandramouli C, Ouwerkerk W, Wander GS, Sawhney JPS, Yap J, MacDonald MR, Ling LH, Sattar N, McMurray JJV, Richards AM, Anand I, Lam CSP. 2019; Microvascular disease in patients with diabetes with heart failure and reduced ejection versus preserved ejection fraction. Diabetes Care. 42:1792–1799. DOI:
10.2337/dc18-2515. PMID:
31292141. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071615771&origin=inward.

9. Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, et al. 2018; Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 20:853–872. DOI:
10.1002/ejhf.1170. PMID:
29520964. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046398177&origin=inward.

12. Ma T, Huang X, Zheng H, Huang G, Li W, Liu X, Liang J, Cao Y, Hu Y, Huang Y. 2021; SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxid Med Cell Longev. 2021:9265016. DOI:
10.1155/2021/9265016. PMID:
34790288. PMCID:
PMC8592716. PMID:
4f884f465e5f482482abd57bf85ffaa8. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85119959525&origin=inward.

19. Chen E, Chen C, Niu Z, Gan L, Wang Q, Li M, Cai X, Gao R, Katakam S, Chen H, Zhang S, Zhou R, Cheng X, Qiu Y, Yu H, Zhu T, Liu J. 2020; Poly(I:C) preconditioning protects the heart against myocardial ischemia/reperfusion injury through TLR3/PI3K/Akt-dependent pathway. Signal Transduct Target Ther. 5:216. DOI:
10.1038/s41392-020-00257-w. PMID:
33154351. PMCID:
PMC7644758. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85095136454&origin=inward.

23. Wang K, Li Z, Sun Y, Liu X, Ma W, Ding Y, Hong J, Qian L, Xu D. 2021; Dapagliflozin improves cardiac function, remodeling, myocardial apoptosis, and inflammatory cytokines in mice with myocardial infarction. J Cardiovasc Transl Res. doi:10.1007/s12265-021-10192-y. DOI:
10.1007/s12265-021-10192-y. PMID:
34855147. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85120464897&origin=inward.
31. Zhang D, Wang X, Tian X, Zhang L, Yang G, Tao Y, Liang C, Li K, Yu X, Tang X, Tang C, Zhou J, Kong W, Du J, Huang Y, Jin H. 2018; The increased endogenous sulfur dioxide acts as a compensatory mechanism for the downregulated endogenous hydrogen sulfide pathway in the endothelial cell inflammation. Front Immunol. 9:882. DOI:
10.3389/fimmu.2018.00882. PMID:
29760703. PMCID:
PMC5936987. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046683013&origin=inward.

32. Han D, Rozanski A, Gransar H, Sharir T, Einstein AJ, Fish MB, Ruddy TD, Kaufmann PA, Sinusas AJ, Miller EJ, Bateman TM, Dorbala S, Di Carli M, Liang JX, Hu LH, Germano G, Dey D, Berman DS, Slomka PJ. 2020; Myocardial ischemic burden and differences in prognosis among patients with and without diabetes: results from the multicenter international REFINE SPECT registry. Diabetes Care. 43:453–459. DOI:
10.2337/dc19-1360. PMID:
31776140. PMCID:
PMC6971784. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85078392590&origin=inward.

33. Shin SH, Claggett B, Pfeffer MA, Skali H, Liu J, Aguilar D, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Lewis EF, Maggioni AP, McMurray JJV, Probstfield JL, Riddle MC, Tardif JC, Solomon SD. 2020; Hyperglycaemia, ejection fraction and the risk of heart failure or cardiovascular death in patients with type 2 diabetes and a recent acute coronary syndrome. Eur J Heart Fail. 22:1133–1143. DOI:
10.1002/ejhf.1790. PMID:
32212368. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082750220&origin=inward.

42. Chiang MH, Liang CJ, Lin LC, Yang YF, Huang CC, Chen YH, Kao HL, Chen YC, Ke SR, Lee CW, Lin MS, Chen YL. 2020; miR-26a attenuates cardiac apoptosis and fibrosis by targeting ataxia-telangiectasia mutated in myocardial infarction. J Cell Physiol. 235:6085–6102. DOI:
10.1002/jcp.29537. PMID:
31990056. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85078656448&origin=inward.

43. Li J, Salvador AM, Li G, Valkov N, Ziegler O, Yeri A, Yang Xiao C, Meechoovet B, Alsop E, Rodosthenous RS, Kundu P, Huan T, Levy D, Tigges J, Pico AR, Ghiran I, Silverman MG, Meng X, Kitchen R, Xu J, et al. 2021; Mir-30d regulates cardiac remodeling by intracellular and paracrine signaling. Circ Res. 128:e1–e23. DOI:
10.1161/CIRCRESAHA.120.317244. PMID:
33092465. PMCID:
PMC7790887. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85100280714&origin=inward.

47. Zhang M, Sui W, Xing Y, Cheng J, Cheng C, Xue F, Zhang J, Wang X, Zhang C, Hao P, Zhang Y. 2021; Angiotensin IV attenuates diabetic cardiomyopathy via suppressing FoxO1-induced excessive autophagy, apoptosis and fibrosis. Theranostics. 11:8624–8639. DOI:
10.7150/thno.48561. PMID:
34522203. PMCID:
PMC8419053. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85112191000&origin=inward.
50. Wu MX, Wang SH, Xie Y, Chen ZT, Guo Q, Yuan WL, Guan C, Xu CZ, Huang YN, Wang JF, Zhang HF, Chen YX. 2021; Interleukin-33 alleviates diabetic cardiomyopathy through regulation of endoplasmic reticulum stress and autophagy via insulin-like growth factor-binding protein 3. J Cell Physiol. 236:4403–4419. DOI:
10.1002/jcp.30158. PMID:
33184863. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85096800458&origin=inward.

52. Han Y, Cai X, Pan M, Gong J, Cai W, Lu D, Xu C. 2022; MicroRNA-21-5p acts via the PTEN/Akt/FOXO3a signaling pathway to prevent cardiomyocyte injury caused by high glucose/high fat conditions. Exp Ther Med. 23:230. DOI:
10.3892/etm.2022.11154. PMID:
35222707. PMCID:
PMC8815051.

55. Chen Q, Zhang L, Chen S, Huang Y, Li K, Yu X, Wu H, Tian X, Zhang C, Tang C, Du J, Jin H. 2016; Downregulated endogenous sulfur dioxide/aspartate aminotransferase pathway is involved in angiotensin II-stimulated cardiomyocyte autophagy and myocardial hypertrophy in mice. Int J Cardiol. 225:392–401. DOI:
10.1016/j.ijcard.2016.09.111. PMID:
27770734. PMID:
https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84992202390&origin=inward.

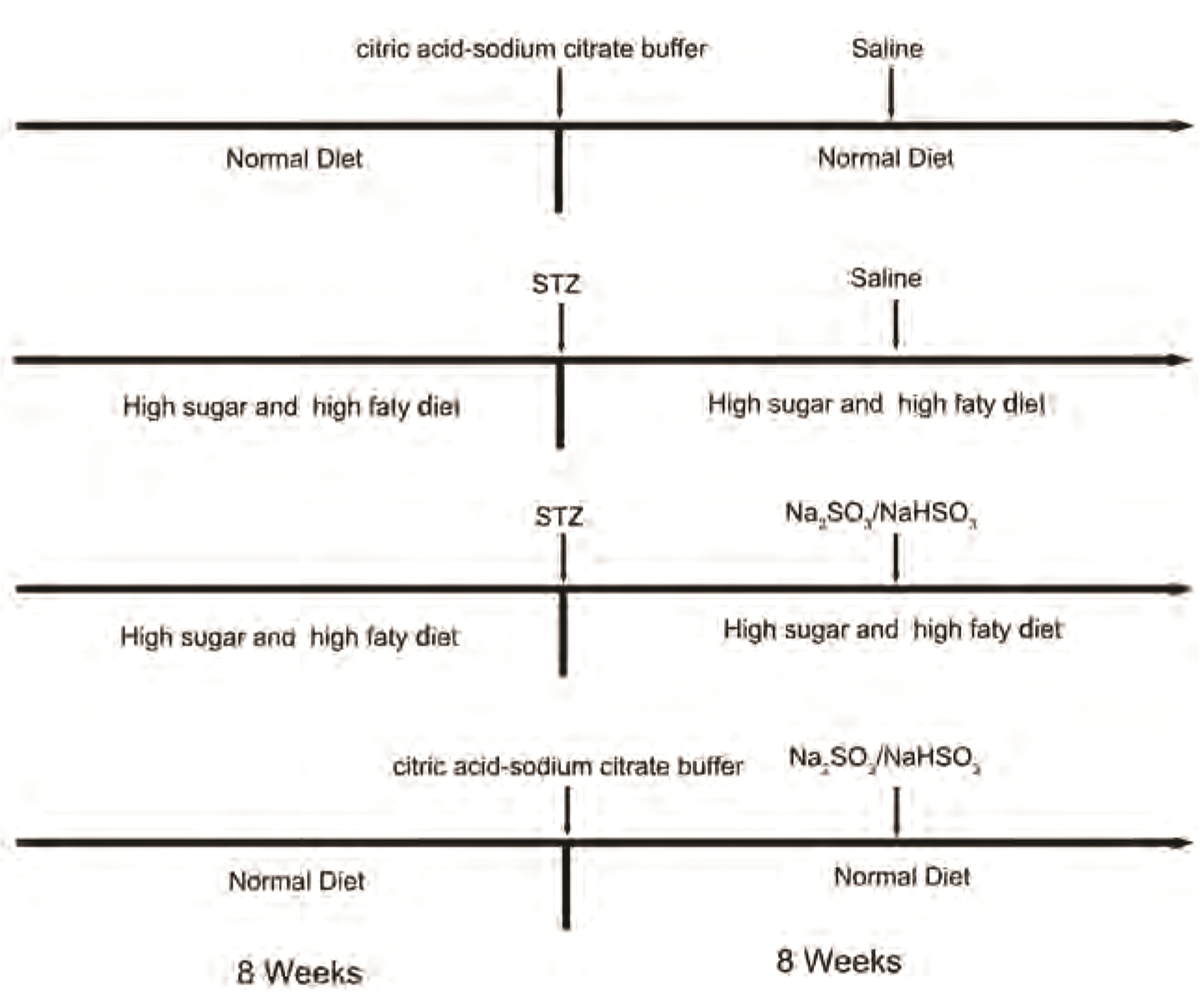










 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download