This article has been corrected. See "Corrigendum to: Thermotherapy as an alternative to exercise for metabolic health in obese postmenopausal women: focus on circulating irisin level" in Volume 27 on page 127.
Abstract
Irisin is a myokine caused by exercise that improves insulin resistance and weight loss. However, under unfavorable conditions such as air pollution, and during the pandemic, outdoor activities are uncomfortable. Therefore, in this study, the effect of heat therapy (half bath 42 ± 0.5°C for 30 min) on irisin circulation levels as an exercise alternative for middle-aged obese women after menopause was investigated. Subjects were 33 women aged 49.54 ± 6.04 years, with parameters of height, 160.12 ± 4.33 cm, weight, 69.71 ± 7.52 kg, body surface area 1.73 ± 0.13 m2, body mass index, 27.19 ± 3.40 kg/m2. The results suggest that circulating irisin levels showed a significant increase after one-time thermotherapy (TH-1). However, the increase in circulating irisin levels after 15 treatments (TH-15, 5 days/week, 3 weeks) was significantly varied. The level of adiponectin, which increases fatty oxidation to reduce fatty deposition, increased significantly at TH-1, but further increased at TH-15, which was significantly different from the level of TH-1. In addition, the basic serum free fatty acid (FFA) level was significantly increased at TH-15 compared to TH-1. Significant differences were also found in the lipid profile (body mass index, waist circumference, and % body fat). Thermotherapy can significantly increase the tympanic temperature and induce changes in circulating irisin and adiponectin levels. Thus, it resulted in positive changes in FFA and lipid profiles. Therefore, repeated thermotherapy is effective in increasing circulating irisin levels in postmenopausal obese women.
Although the average age at which women undergo menopause in Korea has been reported to be approximately 49 years [1], the median age at menopause throughout the world ranges between 46 and 52 years [2]. Considering that life expectancy is gradually increasing, the postmenopausal period can account for > 40% of the life cycle. It is anticipated that there will be 1.1 billion women aged > 50 years worldwide by 2025 [3]. The health challenges associated with metabolic syndrome after menopause is a global concern that transcends countries, regions, and races.
The high incidence of insulin resistance, metabolic syndrome, and cardiovascular diseases in middle-aged women with menopause is closely related to abdominal obesity [4]. In terms of populations (e.g., Asian vs. non-Asians), Asians with a specified body mass index (BMI) carry higher rates of body fat, abdominal obesity, intramyocellular lipids, and liver fat content than Caucasians, which suggests that Asians have a higher insulin resistance even with low BMI [5]. As the secretion of endogenous estrogen after menopause decreases, the prevalence of metabolic syndrome increases [6]. However, treatment with estrogen preparations is not considered a fundamental strategy [7].
Researchers have recently started focusing on obesity-related studies because irisin is an exercise-induced myokine that reduces fat and improves glucose metabolism [8]. Irisin is a proteolytic cleavage product of fibronectin type III domain-containing protein 5 (FNDC5) protein in skeletal muscles [8]. It promotes energy consumption by causing browning of white adipose tissue [9]. Aerobic exercise is effective in increasing the level of circulating irisin in middle-aged and older groups and reducing the amount of systemic fat and abdominal visceral adipose tissue [10]. Therefore, aerobic exercise may be an important strategy to increase FNDC5 gene expression, even in postmenopausal women. Previous study has shown that thermotherapy induces oxidative stress and increases circulating irisin levels [11]. It is known that completely evaporated sweat is equivalent to 580 kcal of heat energy being removed from the body [12-14]. This suggests that thermotherapy has effects similar to exercising.
Exercise environment is important. Although exercise is a strategy to prevent obesity and significantly improve quality of life by enhancing immune system in women after menopause [15,16], aerobic exercise may be limited by the environmental variables and the effects of infectious diseases.
Yale University presented the findings of the Environmental Performance Index in 2018 by setting nine criteria, such as air quality and health consequences, based on 19 indices for 180 countries. Among the three countries in the Far East Asia, Japan ranks relatively higher in at 20th in terms of environmental performance, whereas Korea ranked 60th and China 120th [17]. In a study of 71,431 men in 25 major cities in China, it was found that mortality related to cardio-respiratory diseases increased with exposure to particulate air pollution [18]. According to the levels of national air pollution provided by the Korea Environment Corporation (Air Korea) in real time [19], the number of domestic fine dust (mixture of particles < 10 µm [0.01 mm] from various sources) [20] advisories issued in 17 major cities and provinces, including Seoul, the capital of Korea, is gradually increasing (Supplementary Fig. 1A). Moreover, as the number of hot days increases, the number of people with heat-related illnesses also increases (Supplementary Fig. 1B). There is no universal definition of a heat wave; however, such extreme events associated with hot temperature in summer have been known to impact human mortality [21,22].
In addition, a new coronavirus (COVID-19), which was recently been reported to cause symptoms similar to pneumonia in Wuhan, China, has begun to spread worldwide. The World Health Organization has declared it a pandemic. COVID-19 belongs to a large family of viruses that are known to be transmitted from animals to humans, causing diseases similar to Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). Outdoor workout is limited by because the highly contagious COVID-19, which has spread more rapidly across the world than the combination of SARS and MERS. Thus, recent suggestions have focused on strategies to maintain a healthy lifestyle and to protect public health while minimizing the negative effects of environmental variables [23].
The above environmental health and outdoor environment problems raise the need for research on the effects of thermotherapy. Therefore, this study examined the benefits of repeated thermotherapy (15 times) for circulatory levels of irisin, adiponectin and lipids (BMI, waist circumference and body fat ratio), biomarkers that affect body temperature control in postmenopausal obese women among residents of increasing environmental restrictions.
The subjects were 33 postmenopausal obese women (age, 49.54 ± 6.04 years; height, 160.12 ± 4.33 cm; weight, 69.70 ± 7.52 kg; body surface area, 1.73 ± 0.13 m2; (%) body fat, 32.75 ± 4.06; body mass index, 27.19 ± 3.40 kg/m2; waist size, 85.92 ± 8.10 cm, and tympanic temperature, 36.61 ± 0.31°C) who volunteered to participate in the experiment at the College of Medicine of Soonchunhyang University in Cheonan, Republic of Korea. All participants resided in Chenoan (Republic of Korea), and were nonsmokers, sedentary, and not exposed to estrogen replacement therapy. Subjects were asked to sign the informed consent regarding the study purpose and procedures. All procedures complied with the 2013 Helsinki Declaration of the World Medical Association and approved by the Institutional Review Board on Human Subjects Research and Ethics Committees, Soonchunhyang University (No. 1040875-201611-BR-042), Cheonan, Republic of Korea.
Subjects completed their lunch as usual and were instructed to arrive in the waiting room by 2 pm for the experiment. The core temperature was checked by using a TSK7+1 thermistor probe (Songkitopia, Incheon, Korea) and a K923 small spring (Takara, Yokohama, Japan) before the thermotherapy for 1–15 sessions. The probe was connected to a CF-T1 personal computer (Panasonic, Tokyo, Japan) and a K-720 data logger (Technol Seven, Yokohama, Japan). When the body temperature was 36°C–37°C, subjects were asked to consume 5–7 ml/kg of waterd.
After resting in a sitting position for 60 min in the laboratory, physical parameters including height, weight, BMI, whole body sweat loss volume and percentage of body fat were automatically measured using Inbody equipment (InBody 770; InBody Co., Seoul, Korea). The waist circumference was directly measured by a researcher.
Next, blood was collected, and thermotherapy was applied for 60 min. The subjects who completed thermal treatment were immediately subjected to tympanic temperature (Tty) and blood collection again. Although heat treatment was similarly repeated 15 times, body measurements and blood collection were performed only before (pre) and after (post the first [TH-1] and 15th [TH-15] thermotheraphy. Five-minute breaks in the chamber air temperature were provided every after 5 min of heat loading (Fig. 1) [11]. Intake of alcoholic or caffeine-containing beverages or food and strenuous physical activity was avoided 24 h before the experiment and subjects were asked to sleep for at least 8 h a day during the experiment.
Tty was assessed continuously at 10-sec intervals for 60 min in the left ear by inserting a thermistor probe (TSK7+1; Songkitopia) with a small spring (K923; Takara) connected to a personal computer (CF-T1; Panasonic) and a data logger (K-720; Technol Seven) [24-27]. When the thermistor probe contacted the tympanic membrane, the subject felt slight discomfort and could hear a scratching noise. The inner pinna was filled with small cotton balls to fix the probe in the ear [11,24-27].
Skin temperatures of the chest (Tschest), upper arm (Tsarm), thigh (Tsthigh), and leg (Tsleg) were measured (continuous assessment, Fig. 1) with a TSK7+1 thermistor probe (Songkitopia) and a K923 small spring (Takara) [24-27]. The probe was connected to a CF-T1 personal computer (Panasonic) and a K-720 data logger (Technol Seven). Mean skin temperature (mTs) was calculated using the Ramanathan equation [28]. The parameter mTb was calculated using the formula of Sugenoya and Ogawa [29]: mTb = (0.9·Tty + 0.1·mTs).
Thermotherapy was conducted in the city of Cheonan (Chungnam), Republic of Korea. Cheonan is located in the southwest part of Korea (126°52´N, 33.38´E) and extends to the northeast (130°4´N, 43.0´E). The test conditions were maintained at 26.5 ± 0.3°C and relative humidity of 60 ± 0. 3.0% with an air velocity of 1 m/s. Thermotherapy involved immersing half the body (up to the belly button) into a hot water bath at a temperature of 42 ± 0.5°C for 60 min similar to conditions reported in previous studies [26,30,31]. However, considering that the subjects were middle-aged, continuous thermotherapy for 30 min was avoided. Although the thermotherapy was conducted for 60 min, the treatment was stopped every 5 min, and the subjects were instructed to emerge out of the hot water bath and rest for 5 min. A 5-min break was provided after 5, 10, 15, 20, 25, and 30 min of heat loading to minimize possible discomfort during physical immersion. Additionally, a drinkable 500 ml of tepid (25.0 ± 0.5°C) water bottle was provided for each subjects to prevent dehydration (Fig. 1) [11]. Thus, the actual heat loading was applied for 30 min, and all subjects were exposed to 15 rounds of treatment (5 day/week, for 3 weeks) (conducted once a day and every other day). The experimental period ranged from January 3, 2018 to February 2, 2018 (Supplementary Fig. 2). All subjects wore shorts and short-sleeved shirts provided by the researchers.
Before measuring WC, subjects were made to stand upright while breathing lightly. In this posture, the waist size was measured with a tapeline held horizontally at the mid-portion between lowermost part of rib and uppermost part of iliac crest. Care was taken not to press the skin. WC was measured to the nearest 0.1 cm by a single experienced researcher [32].
Subjects were asked to fast for 12 h and sleep for at least 8 h the night before testing.
The protocol consisted of 10 min of bed rest in the supine position followed by analysis of expired gas samples for 30 min. A computerized gas analyzer (Quark PFT; Cosmed, Rome, Italy) was used to determine oxygen uptake (VO2) and carbon dioxide output (VCO2). BMR was calculated by following formula; [(3.9 ×VO2) + (1.1 × VCO2)] × 1,440 [33].
Body weight loss after half-body immersion can be caused by sweating. Body weight was therefore measured before and after immersion with a precision instrument (IW2, Sartorius, Germany) to determine the whole-body sweat loss volume [31].
Blood samples were collected at before and after experiment in TH-1 and TH-15. Blood samples were collected in a serum separator tube from the antecubital vein and left at room temperature for 15–30 min to enable clotting, after which the samples were centrifuged at 3,000 rpm (2,000 ×g) and 4°C for 10 min. Serum was then harvested and stored at −80°C until analysis. Irisin was determined using a commercial ELISA kit (Irisin EIA kit EK-067–16; Phoenix Pharmaceuticals, Burlingame, CA, USA) with a spectrophotometric reader. Adiponectin was measured using a commercial Microplate Reader (USA) ELISA with a spectrophotometric reader. Serum free fatty acids (FFA) were analyzed using a model 7180 automated analyzer (Hitachi, Tokyo, Japan).
Descriptive statistics are expressed as mean ± standard deviation (SD) using the commercially-available computer software SPSS for Windows, version 21.0 (IBM Co., Armonk, NY, USA). Normality of the data were verified via Shapiro–Wilk normality test followed by calculating skewness and kurtosis of the statistics. Statistical significance was determined using student t-test and paired t-test to conduct a comparison between TH-1 (Pre vs. Post) and TH-15 (Pre vs. Post) heat stimulation. Linear regression analysis and Pearson correlation coefficient were used to assess the correlations between variables. Significant differences were considered at p < 0.05.
Tty increased significantly in TH-1 (Pre, 36.61 ± 0.31°C vs. Post, 38.48 ± 0.33°C) and TH-15 (Pre, 36.79 ± 0.33 vs. Post, 38.75 ± 0.34°C) (***p < 0.001). Particularly, Tty after TH-15 was significantly higher than TH-1 (Pre, △TR rest = 0.18°C, #p < 0.05; Post, △TP post = 0.27°C, #p < 0.05) (Fig. 2A).
As shown in Fig. 2B, BMRs were higher in TH-15 than in TH-1 before (##p < 0.01) and after (###p < 0.001) the heat load test, respectively. After heat load, BMRs were increased by 13.35 kcal/kg/day (***p < 0.001) in TH-1 and 16.91 kcal/kg/day (***p < 0.001) in TH-15.
Correlation between Tty and BMR before and after half-body immersion protocol is shown in Fig. 3. A positive relationship between Tty and BMR was observed before (R2 = 0.671, ***p < 0.001) and after (R2 = 0.664, ***p < 0.001) hot water treatment was also observed.
The levels of circulating irisin increased significantly after thermotherapy (TH-1, Pre, 6.05 ± 1.59 ng/ml vs. Post, 7.92 ± 1.82 ng/ml, ***p < 0.001; TH-15, Pre, 7.24 ± 1.44 ng/ml vs. Post, 9.65 ± 2.01 ng/ml, ***p < 0.001). The circulating irisin levels before and after TH-15 showed significant difference compared with that of TH-1, respectively (Pre, ##p < 0.01; Post, ##p < 0.01) (Fig. 4).
Correlation between Tty and irisin levels before and after half-body immersion protocol is shown in Fig. 5. A positive relationship between Tty and irisin levels was observed before (R2 = 0.653, **p < 0.01) and after (R2 = 0.653, **p < 0.01) hot water treatment was also observed.
The levels of circulating adiponectin increased significantly after thermotherapy (TH-1; Pre, 8.13 ± 2.80 μg/ml vs. Post, 10.24 ± 2.85 μg/ml, ***p < 0.001; TH-15; Pre, 9.57 ± 2.47 μg/ml vs. Post, 11.86 ± 2.76 μg/ml, ***p < 0.001), and the levels of circulating adiponectin after TH-15 showed a significant difference compared with those after TH-1 (Pre, ##p < 0.01; Post, ##p < 0.01) (Fig. 6).
The levels of circulating FFA increased significantly after thermotherapy (TH-1; Pre, 467.15 ± 148.96 μEq/L vs. Post, 551.64 ± 164.53 μEq/L, ***p < 0.001; TH-15; Pre, 532.38 ± 124.37 μEq/L vs. Post, 627.25 ± 149.28 μEq/L, ***p < 0.001), and the levels of circulating FFA after TH-15 showed a significant difference compared with those after TH-1 (Pre, ##p < 0.01; Post, ##p < 0.01) (Fig. 7).
As shown in Fig. 8, subjects in TH-1 lost a total sweat output of 564 ± 208 ml, wheras those after TH-15 lost a total sweat output of 712 ± 225 ml. The difference in whole body sweat loss volume between the TH-1 and TH-15 (increase rate of 26.24%) was statistically significant (###p < 0.001).
The levels of BMI decreased significantly after thermotherapy (TH-1, Pre vs. Post, *p < 0.05; TH-15, Pre vs. Post, **p < 0.01), and there was a significant difference between TH-15 and TH-1 (Pre, #p < 0.05; Post, ###p < 0.001). WC significantly decreased after thermotherapy (TH-1, Pre vs. Post, **p < 0.01; TH-15, Pre vs. Post, ***p < 0.001), and showed a significant difference between the two treatments (Pre, #p < 0.05; Post, ###p < 0.001). The percentage of body fat also decreased significantly after thermotherapy (TH-1, Pre vs. Post, *p < 0.05; TH-15, Pre vs. Post, ***p < 0.001), with a significant difference between the two treatments (Pre, ##p < 0.01; Post, ###p < 0.001) (Table 1). Thus, single exposure to TH-1 resulted in significantly reduced obesity indices.
Irisin is a substance released from muscle tissue in response to exercise and low-temperature environments [34]. It improves insulin resistance and reduces weight [8]. However, considering the recent environmental factors in Korea, outdoor exercises are restricted to obese women after menopause. Half bath is a type of bath culture popular among Koreans, and its application is simple. Hence, this study used thermotherapy via repeated half-bath using hot water as a strategy to replace exercise and minimize fat accumulation in obese women after menopause. Furthermore, it has been already known that high temperatures lead to oxidative stress [35]. Recently, study with women subjects has shown that thermotherapy can upregulate circulating irisin level by inducing oxidative stress [11]. In our study, it can be seen that the circulating irisin level rose before and after treatment during the first thermotherapy (TH-1, Pre, 6.05 ± 1.59 ng/ml vs. Post, 7.92 ± 1.82 ng/ml, ***p < 0.001), and the base line increased during the last thermotherapy (TH-15, Pre, 7.24 ± 1.44 ng/ml vs. Post, 9.65 ± 2.01 ng/ml, ***p < 0.001, Fig. 4). Increment of oxidative stress markers that elevated after thermotherapy were positively related with the increase in circulating irisin concentration. This is consistent with findings of Samy et al. [36] that muscle damage and oxidative stress were related to irisin concentration. The result of this study showed that the positive relationships between the concentration of irisin and Tty [11] (**p < 0.01, Fig. 5). Therefore, this study suggests that thermotherapy induces oxidative stress in middle-aged women after menopause, resulting in increased levels of circulating irisin. This study found that the levels of circulating irisin, adiponectin, and FFA increased significantly after repeated thermotherapy (15 sessions) for 3 weeks. In addition, the significant increase in sweat volume loss of 26.24% in TH-15 (712 ± 225 ml) compared to TH-1 (564 ± 208 ml) explains that thermotherapy resulted in increased basal metabolism and similar energy consumption to exercise [14]. Therefore, in this study, BMI, WC and percentage of body fat significantly decreases similar to exercise during thermal therapy. Despite the several differences depending on the type of exercise, exercise in general induces the expression of irisin/FNDC5 [8,37], which is strongly related to modulate the physiological or pathophysiological response to environmental, metabolic, emotional, and psychological stimuli [38]. From this point of view, thermotherapy also increased circulating irisin levels to respond to oxidative stress, similar to exercise [11]. Thermotherapy is valuable as an alternative treatment for menopause women due to a decrease in the amount of exercise due to restrictions on outdoor activities. The major factors contributing to excessive fat accumulation in middle-aged women are aging, decreased physical activity, and reduced muscle mass (sarcopenia), which decrease the metabolic rate [39-41]. Adipose tissue not only stores lipids but also functions as a paracrine and endocrine organ. It also secretes adiponectin to promote fat oxidation in skeletal muscles and inhibits hepatic glucose production to improve systemic energy homeostasis [42]. Furthermore, it reduces inflammation in various cells, such as those in the vasculature, heart, lung, and colon, via AdipoR1 and R2 receptor signaling, anti-inflammatory and anti-apoptotic mechanisms [43]. Because adiponectin enhances lipid profile [44], it is also used as an objective index to explain the correlation between central obesity and insulin resistance [45]. Our study also confirmed an increase in adiponectin during thermotherapy, which can be seen that thermotherapy leads to metabolic improvement (TH-1; Pre, 8.13 ± 2.80 μg/ml vs. Post, 10.24 ± 2.85 μg/ml, ***p < 0.001; TH-15; Pre, 9.57 ± 2.47 μg/ml vs. Post, 11.86 ± 2.76 μg/ml, ***p < 0.001, Fig. 6). A significant increase in adiponectin depending on environmental conditions may be a predictor of increased activity of brown adipose tissue [46]. In addition, the levels of plasma adiponectin decrease in obesity and diabetes [47].
Therefore, repeated thermotherapy in postmenopausal middle-aged women was effective in increasing the levels of circulating irisin and adiponectin and in improving lipid profile. Studies investigated the preventive effects of bathing against hypertension, hyperlipidemia, and depression [48-50]. However, this study is the first of its kind to measure the levels of circulating irisin and to show that thermotherapy can be an alternative to exercise in increasing the levels of circulating irisin.
Thermotherapy is economical, and unlike medical treatment, it is a preventive concept and is meaningful in that it is highly accessible and convenient. Based on the individual physical characteristics, lifestyle, and presence of disease, thermotherapy can be implemented under various conditions. Repeated thermotherapy promotes cyclical irisin activation, so intermittent treatment is recommended rather than continuous treatment.
Supplementary data including one table and two figures can be found with this article online at https://doi.org/10.4196/kjpp.2022.26.6.501.
Notes
REFERENCES
1. Yang HJ, Suh PS, Kim SJ, Lee SY. 2015; Effects of smoking on menopausal age: results from the Korea National Health and Nutrition Examination Survey, 2007 to 2012. J Prev Med Public Health. 48:216–224. DOI: 10.3961/jpmph.15.021. PMID: 26265667. PMCID: PMC4542296. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84938827154&origin=inward.

2. Amiri P, Deihim T, Nakhoda K, Hasheminia M, Montazeri A, Azizi F. 2014; Metabolic syndrome and health-related quality of life in reproductive age and post-menopausal women: Tehran Lipid and Glucose Study. Arch Iran Med. 17:423–428. PMID: 24916528. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84902526881&origin=inward.
3. Nappi RE, Palacios S. 2014; Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric. 17:3–9. DOI: 10.3109/13697137.2013.871696. PMID: 24423885. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84892737699&origin=inward.

4. Goh VHH, Hart WG. 2018; Excess fat in the abdomen but not general obesity is associated with poorer metabolic and cardiovascular health in premenopausal and postmenopausal Asian women. Maturitas. 107:33–38. DOI: 10.1016/j.maturitas.2017.10.002. PMID: 29169577. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030837752&origin=inward.

5. Wulan SN, Westerterp KR, Plasqui G. 2010; Ethnic differences in body composition and the associated metabolic profile: a comparative study between Asians and Caucasians. Maturitas. 65:315–319. DOI: 10.1016/j.maturitas.2009.12.012. PMID: 20079586. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77649180244&origin=inward.

6. Mauvais-Jarvis F, Clegg DJ, Hevener AL. 2013; The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 34:309–338. DOI: 10.1210/er.2012-1055. PMID: 23460719. PMCID: PMC3660717. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84878632677&origin=inward.

7. Palmisano BT, Zhu L, Eckel RH, Stafford JM. 2018; Sex differences in lipid and lipoprotein metabolism. Mol Metab. 15:45–55. DOI: 10.1016/j.molmet.2018.05.008. PMID: 29858147. PMCID: PMC6066747. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85047506095&origin=inward.

8. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. 2012; A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 481:463–468. DOI: 10.1038/nature10777. PMID: 22237023. PMCID: PMC3522098. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84862776702&origin=inward.

9. Rodríguez A, Becerril S, Ezquerro S, Méndez-Giménez L, Frühbeck G. 2017; Crosstalk between adipokines and myokines in fat browning. Acta Physiol (Oxf). 219:362–381. DOI: 10.1111/apha.12686. PMID: 27040995. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85010850003&origin=inward.

10. Miyamoto-Mikami E, Sato K, Kurihara T, Hasegawa N, Fujie S, Fujita S, Sanada K, Hamaoka T, Tabata I, Iemitsu M. 2015; Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLoS One. 10:e0120354. DOI: 10.1371/journal.pone.0120354. PMID: 25793753. PMCID: PMC4368602. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84925779021&origin=inward.

11. Park TH, Lee HJ, Lee JB. 2021; Effect of heat stimulation on circulating irisin in humans. Front Physiol. 12:675377. DOI: 10.3389/fphys.2021.675377. PMID: 34262475. PMCID: PMC8273865. PMID: 6976cbe75b804346b69b026449b67bbc. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85109879766&origin=inward.

12. Wenger CB. 1972; Heat of evaporation of sweat: thermodynamic considerations. J Appl Physiol. 32:456–459. DOI: 10.1152/jappl.1972.32.4.456. PMID: 5026491. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0015324876&origin=inward.

13. Sawka MN, Leon LR, Montain SJ, Sonna LA. 2011; Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol. 1:1883–1928. DOI: 10.1002/cphy.c100082. PMID: 23733692. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84857658040&origin=inward.

14. Baker LB. 2017; Sweating rate and sweat sodium concentration in athletes: a review of methodology and intra/interindividual variability. Sports Med. 47(Suppl 1):111–128. DOI: 10.1007/s40279-017-0691-5. PMID: 28332116. PMCID: PMC5371639. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85015929812&origin=inward.

15. de Araújo AL, Silva LC, Fernandes JR, Benard G. 2013; Preventing or reversing immunosenescence: can exercise be an immunotherapy? Immunotherapy. 5:879–893. DOI: 10.2217/imt.13.77. PMID: 23902557. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84881290703&origin=inward.

16. Baker A, Sirois-Leclerc H, Tulloch H. 2016; The impact of long-term physical activity interventions for overweight/obese postmenopausal women on adiposity indicators, physical capacity, and mental health outcomes: a systematic review. J Obes. 2016:6169890. DOI: 10.1155/2016/6169890. PMID: 27293882. PMCID: PMC4884891. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84973364653&origin=inward.

17. Wendling ZA, Emerson JW, Esty DC, Levy MA, de Sherbinin A. 2018. 2018 Environmental performance index. Yale Center for Environmental Law & Policy;New Haven: https://epi.yale.edu/downloads/epi2018reportv06191901.pdf. DOI: 10.2217/imt.13.77. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84881290703&origin=inward.
18. Zhou M, Liu Y, Wang L, Kuang X, Xu X, Kan H. 2014; Particulate air pollution and mortality in a cohort of Chinese men. Environ Pollut. 186:1–6. DOI: 10.1016/j.envpol.2013.11.010. PMID: 24333659. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84890191832&origin=inward.

19. AirKorea. Fine dust [Internet]. AirKorea;Incheon: Available from: https://www.airkorea.or.kr/web/pmWarning?pMENU_NO=115. cited 2020 Jul 1.
20. Ritz B, Hoffmann B, Peters A. 2019; The effects of fine dust, ozone, and nitrogen dioxide on health. Dtsch Arztebl Int. 51-52:881–886. DOI: 10.3238/arztebl.2019.0881. PMID: 31941576. PMCID: PMC6976917. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85077941113&origin=inward.

21. Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. 2000; Climate extremes: observations, modeling, and impacts. Science. 289:2068–2074. DOI: 10.1126/science.289.5487.2068. PMID: 11000103. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034703308&origin=inward.

22. Meehl GA, Tebaldi C. 2004; More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 305:994–997. DOI: 10.1126/science.1098704. PMID: 15310900. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=4043154304&origin=inward.

23. Slezakova K, Pereira MC, Morais S. 2020; Ultrafine particles: levels in ambient air during outdoor sport activities. Environ Pollut. 258:113648. DOI: 10.1016/j.envpol.2019.113648. PMID: 31806467. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85076231069&origin=inward.

24. Lee JB, Kim TW, Min YK, Yang HM. 2013; Seasonal acclimatization to the hot summer over 60 days in the Republic of Korea suppresses sweating sensitivity during passive heating. J Therm Biol. 38:294–299. https://doi.org/10.1016/j.jtherbio.2013.03.006. DOI: 10.1016/j.jtherbio.2013.03.006. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84877023854&origin=inward.

25. Shin YO, Lee JB, Min YK, Yang HM. 2013; Heat acclimation affects circulating levels of prostaglandin E2, COX-2 and orexin in humans. Neurosci Lett. 542:17–20. DOI: 10.1016/j.neulet.2013.03.017. PMID: 23523649. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84876744845&origin=inward.

26. Lee J, Shin Y. 2017; Comparison of density and output of sweat gland in tropical Africans and temperate Koreans. Auton Neurosci. 205:67–71. DOI: 10.1016/j.autneu.2017.05.004. PMID: 28506659. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85019094091&origin=inward.

27. Lee JB, Kim JH. 2018; Decreased thermal sweating of central sudomotor mechanism in African and Korean men. Am J Hum Biol. 30:e23091. DOI: 10.1002/ajhb.23091. PMID: 29341311. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85047994485&origin=inward.

28. Ramanathan NL. 1964; A new weighting system for mean surface temperature of the human body. J Appl Physiol. 19:531–533. DOI: 10.1152/jappl.1964.19.3.531. PMID: 14173555. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=14944349971&origin=inward.

29. Sugenoya J, Ogawa T. 1985; Characteristics of central sudomotor mechanism estimated by frequency of sweat expulsions. Jpn J Physiol. 35:783–794. DOI: 10.2170/jjphysiol.35.783. PMID: 4079134. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0022394986&origin=inward.

30. Kim TW, Lee JB. 2013; The effects of caffeine ingestion before passive heat loading on serum leptin levels in humans. Appl Biochem Biotechnol. 171:1253–1261. DOI: 10.1007/s12010-013-0296-x. PMID: 23709289. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84888129274&origin=inward.

31. Lee JB, Kim TW. 2015; Increased levels of FFA during passive heat loading after a 2-week repeated heat load in Koreans. Int J Biometeorol. 59:473–475. DOI: 10.1007/s00484-014-0849-x. PMID: 24865598. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84939895547&origin=inward.

32. Kim TW, Shin YO, Lee JB, Min YK, Yang HM. 2010; Effect of caffeine on the metabolic responses of lipolysis and activated sweat gland density in human during physical activity. Food Sci Biotechnol. 19:1077–1081. https://link.springer.com/article/10.1007/s10068-010-0151-6. DOI: 10.1007/s10068-010-0151-6. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79957650578&origin=inward.

33. Weir JB. 1949; New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 109:1–9. DOI: 10.1113/jphysiol.1949.sp004363. PMID: 15394301. PMCID: PMC1392602. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=8944256349&origin=inward.

34. Lee JB, Kim TW. 2014; Passive heat loading links lipolysis and regulation of fibroblast growth factor-21 in humans. J Therm Biol. 45:163–167. DOI: 10.1016/j.jtherbio.2014.09.004. PMID: 25436966. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84907553418&origin=inward.

35. Pörtner HO. 2001; Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 88:137–146. DOI: 10.1007/s001140100216. PMID: 11480701. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034978152&origin=inward.

36. Samy DM, Ismail CA, Nassra RA. 2015; Circulating irisin concentrations in rat models of thyroid dysfunction -- effect of exercise. Metabolism. 64:804–813. DOI: 10.1016/j.metabol.2015.01.001. PMID: 25720940. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929654073&origin=inward.

37. Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS. 2013; Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One. 8:e63517. DOI: 10.1371/journal.pone.0063517. PMID: 23667629. PMCID: PMC3646740. PMID: c6eb509b4526482e856d21e796d282c2. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84877149547&origin=inward.

38. Brindley RL, Bauer MB, Blakely RD, Currie KPM. 2017; Serotonin and serotonin transporters in the adrenal medulla: a potential hub for modulation of the sympathetic stress response. ACS Chem Neurosci. 8:943–954. DOI: 10.1021/acschemneuro.7b00026. PMID: 28406285. PMCID: PMC5541362. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85019610825&origin=inward.

39. Sternfeld B, Wang H, Quesenberry CP Jr, Abrams B, Everson-Rose SA, Greendale GA, Matthews KA, Torrens JI, Sowers M. 2004; Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women's Health Across the Nation. Am J Epidemiol. 160:912–922. DOI: 10.1093/aje/kwh299. PMID: 15496544. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=7244253095&origin=inward.

40. Karakelides H, Nair KS. 2005; Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 68:123–148. DOI: 10.1016/S0070-2153(05)68005-2. PMID: 16124998. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=25144501166&origin=inward.

41. Kuk JL, Saunders TJ, Davidson LE, Ross R. 2009; Age-related changes in total and regional fat distribution. Ageing Res Rev. 8:339–348. DOI: 10.1016/j.arr.2009.06.001. PMID: 19576300. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=69049093712&origin=inward.

42. Ahima RS, Flier JS. 2000; Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 11:327–332. DOI: 10.1016/S1043-2760(00)00301-5. PMID: 10996528. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033789518&origin=inward.

43. Fang H, Judd RL. 2018; Adiponectin regulation and function. Compr Physiol. 8:1031–1063. DOI: 10.1002/cphy.c170046. PMID: 29978896. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85050621609&origin=inward.

44. Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. 2003; Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 289:1799–1804. DOI: 10.1001/jama.289.14.1799. PMID: 12684358. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037840403&origin=inward.

45. Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. 2003; Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 88:4823–4831. DOI: 10.1210/jc.2003-030214. PMID: 14557461. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0242288690&origin=inward.

46. Sun L, Yan J, Goh HJ, Govindharajulu P, Verma S, Michael N, Sadananthan SA, Henry CJ, Velan SS, Leow MK. 2020; Fibroblast growth factor-21, leptin, and adiponectin responses to acute cold-induced brown adipose tissue activation. J Clin Endocrinol Metab. 105:e520–e531. DOI: 10.1210/clinem/dgaa005. PMID: 31912874. PMCID: PMC7015460. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85079356511&origin=inward.

47. Toczylowski K, Hirnle T, Harasiuk D, Zabielski P, Lewczuk A, Dmitruk I, Ksiazek M, Sulik A, Gorski J, Chabowski A, Baranowski M. 2019; Plasma concentration and expression of adipokines in epicardial and subcutaneous adipose tissue are associated with impaired left ventricular filling pattern. J Transl Med. 17:310. DOI: 10.1186/s12967-019-2060-7. PMID: 31533725. PMCID: PMC6751580. PMID: 021fb97085e549cb9cb651d42d29b10e. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85072374784&origin=inward.

48. Oláh M, Koncz A, Fehér J, Kálmánczhey J, Oláh C, Balogh S, Nagy G, Bender T. 2010; The effect of balneotherapy on C-reactive protein, serum cholesterol, triglyceride, total antioxidant status and HSP-60 levels. Int J Biometeorol. 54:249–254. DOI: 10.1007/s00484-009-0276-6. PMID: 19937457. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77951769044&origin=inward.

49. Ishikawa J, Yoshino Y, Watanabe S, Harada K. 2016; Reduction in central blood pressure after bathing in hot water. Blood Press Monit. 21:80–86. DOI: 10.1097/MBP.0000000000000167. PMID: 26657048. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84949895347&origin=inward.

50. Janssen CW, Lowry CA, Mehl MR, Allen JJ, Kelly KL, Gartner DE, Medrano A, Begay TK, Rentscher K, White JJ, Fridman A, Roberts LJ, Robbins ML, Hanusch KU, Cole SP, Raison CL. 2016; Whole-body hyperthermia for the treatment of major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 73:789–795. DOI: 10.1001/jamapsychiatry.2016.1031. PMID: 27172277. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84982792300&origin=inward.

Fig. 1
Schematic representation of the experimental protocol condition in air temperature 25.0 ± 0.5°C and relative humidity 60.0 ± 3.0%.
Heat stimulation entailed immersing half of the body into a hot water bath (42 ± 0.5°C) for total 30 min and break for total 30 min. Blood sampling was done before immersion (Pre) and immediately after the last break (Post). Tympanic and local skin temperature measurements were conducted continuously at 10-sec intervals for 60 min.
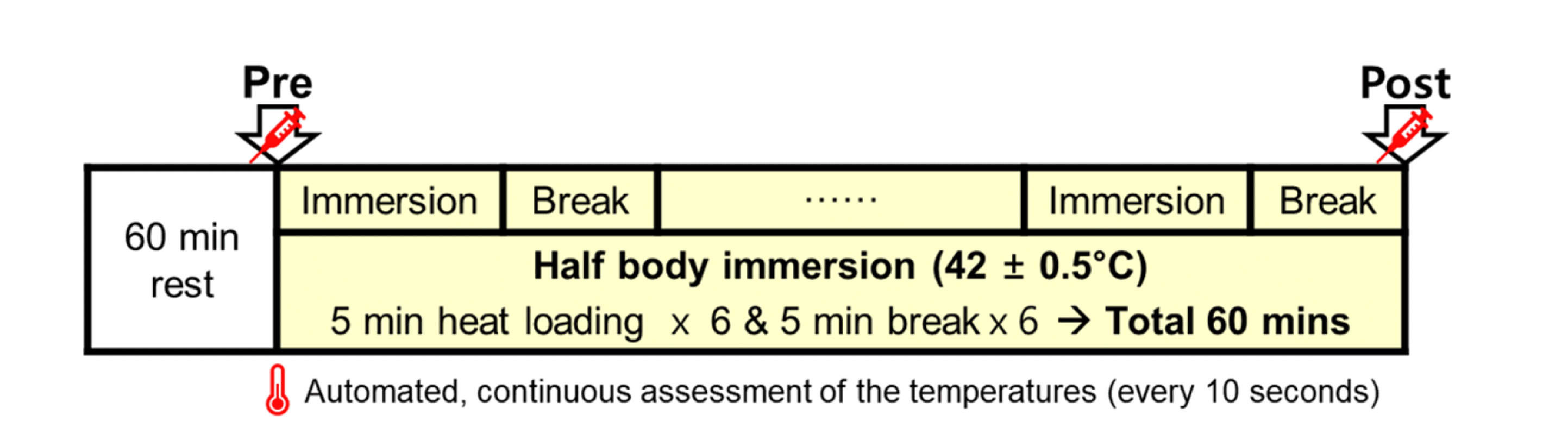
Fig. 2
Tympanic temperature and basal metabolic rate.
(A) Tympanic temperature, Pre (white circle) and Post (black circle), (B) basal metabolic rate (BMR), Pre (white bar) and Post (black bar) on thermotherapy. TH-1 (pre □: 28.27 ± 8.06 kcal/kg/day vs. post ■: 41.62 ± 9.13 kcal/kg/day, ***p < 0.001) and TH-15 (pre □: 32.41 ± 9.52 kcal/kg/day vs. post ■: 49.32 ± 10.64 kcal/kg/day, ***p < 0.001) for differences between Pre vs. Post. #p < 0.01, ##p < 0.01, and ###p < 0.001 for difference between TH-1 and TH-15. Values (n = 33) are mean ± SD. Resting BMR of TH-15 was significantly higher than in TH-1 (before thermotherapy, 28.27 ± 8.06 kcal/kg/day vs. 41.62 ± 9.13 kcal/kg/day, ##p < 0.01). BMR was increased by 13.35 kcal/kg/day (***p < 0.001) in TH-1 and by 16.91 kcal/kg/day (***p < 0.001) in TH-15. TH, thermotherapy, immersing half-bath, 42 ± 0.5°C for 60 min (5-min break at 5-min intervals); TH-1, application of thermotherapy once; TH-15, application of thermotherapy repeated 15 times.
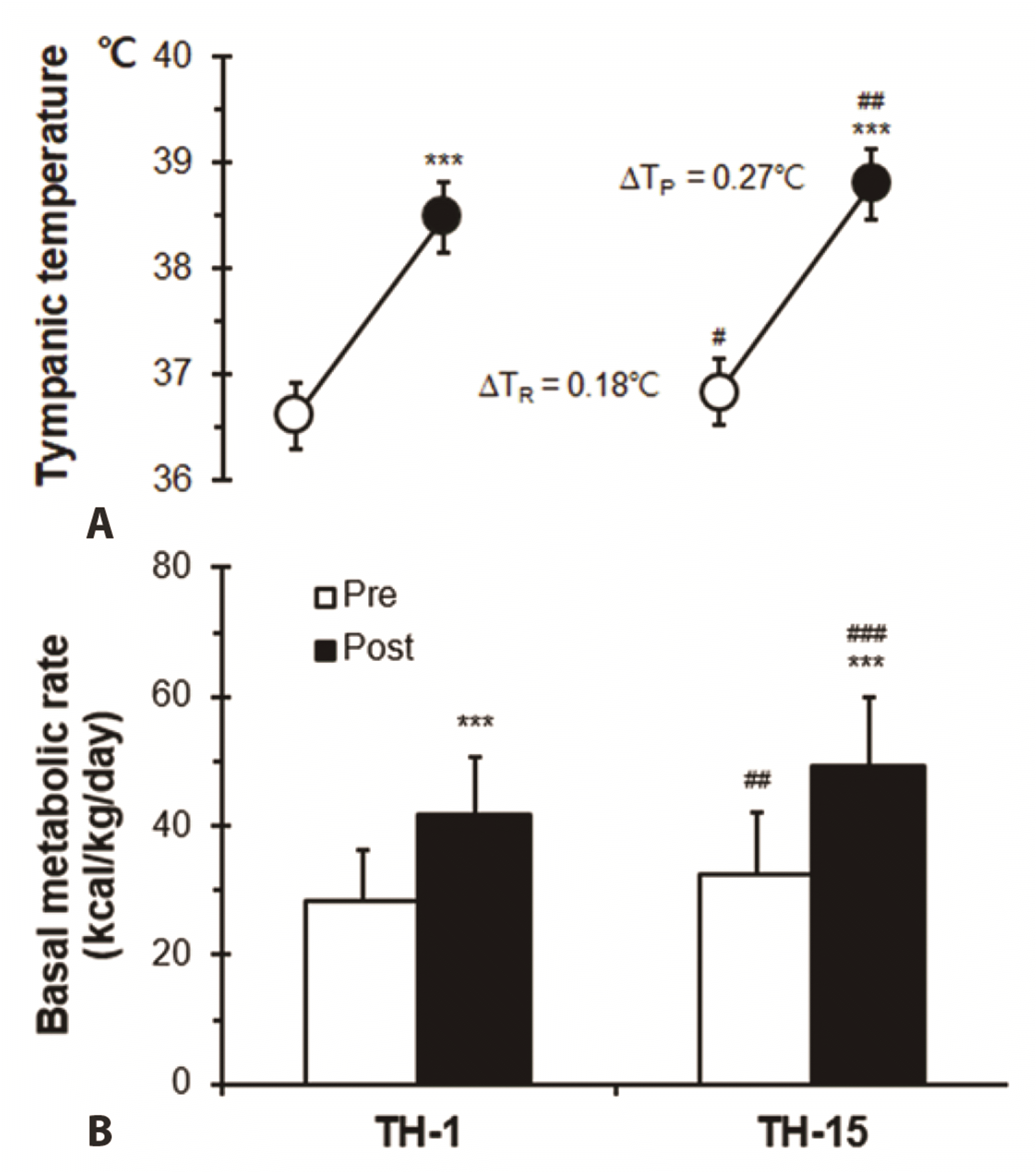
Fig. 3
Correlation between the tympanic temperature and basal metabolic rate (BMR) before (Pre; ◦) and after (Post; •) hot water bath (42 ± 0.5°C) immersion at TH-15.
TH, thermotherapy. ***p < 0.001, statistically significant difference (Pre; R2 = 0.671, Post; R2 = 0.664).
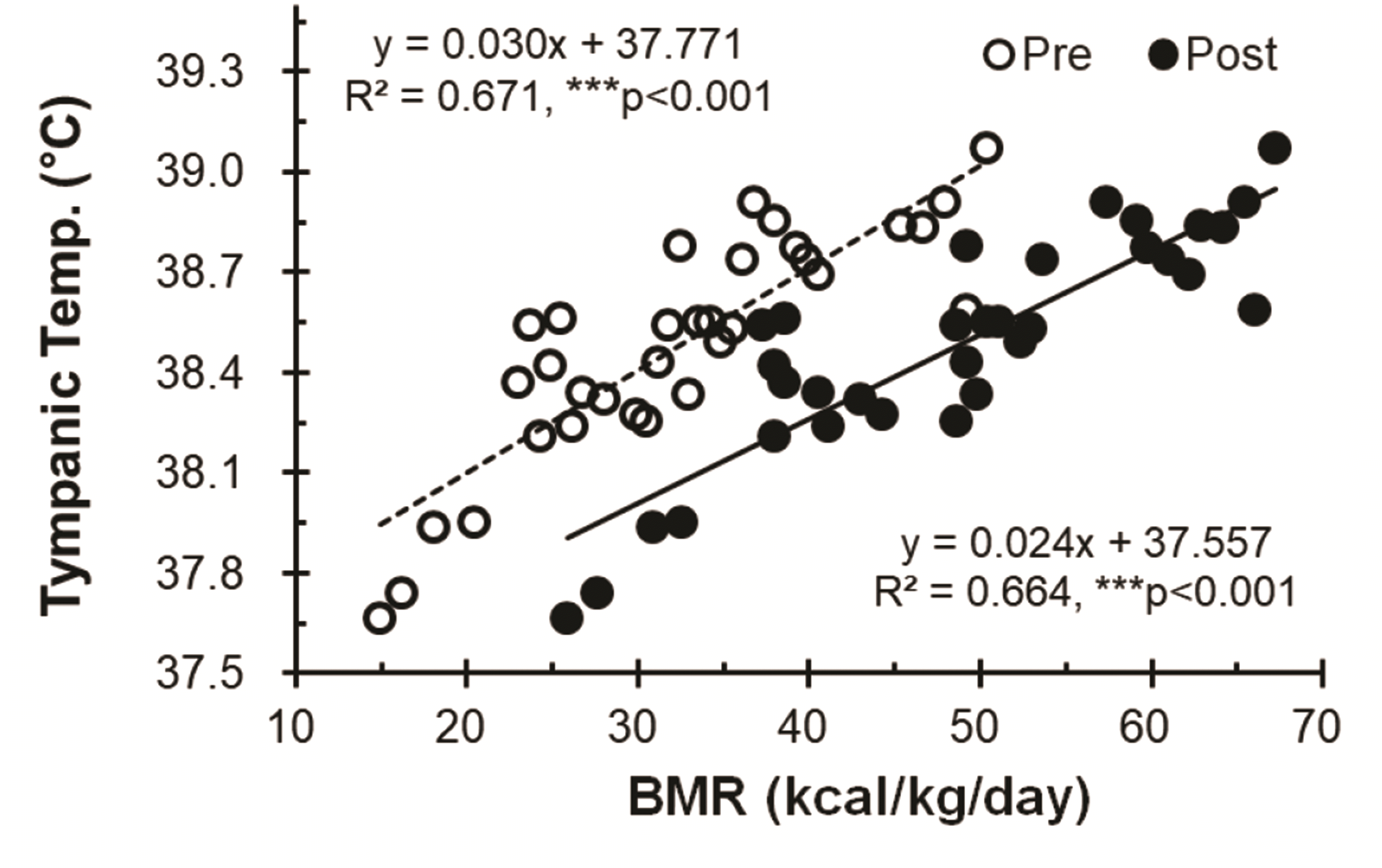
Fig. 4
Changes in the levels of irisin following thermotherapy.
Values (n = 33) are mean ± SD. Changes in the levels of circulating irisin. Statistically significant differences were found between Pre and Post, ***p < 0.001, and significant differences betweenTH-1 and TH-15, ##p < 0.01. TH, thermotherapy, immersing half-bath, 42 ± 0.5°C for 60 min (5-min break at 5-min intervals); TH-1, application of thermotherapy once; TH-15, application of thermotherapy repeated 15 times.
Fig. 5
Correlation between the tympanic temperature and irisin concentration before (Pre; ◦) and after (Post; •) hot water bath (42 ± 0.5°C) immersion at TH-15.
TH, thermotherapy. **p < 0.01, statistically significant difference (Pre; R2 = 0.653, Post; R2 = 0.653).
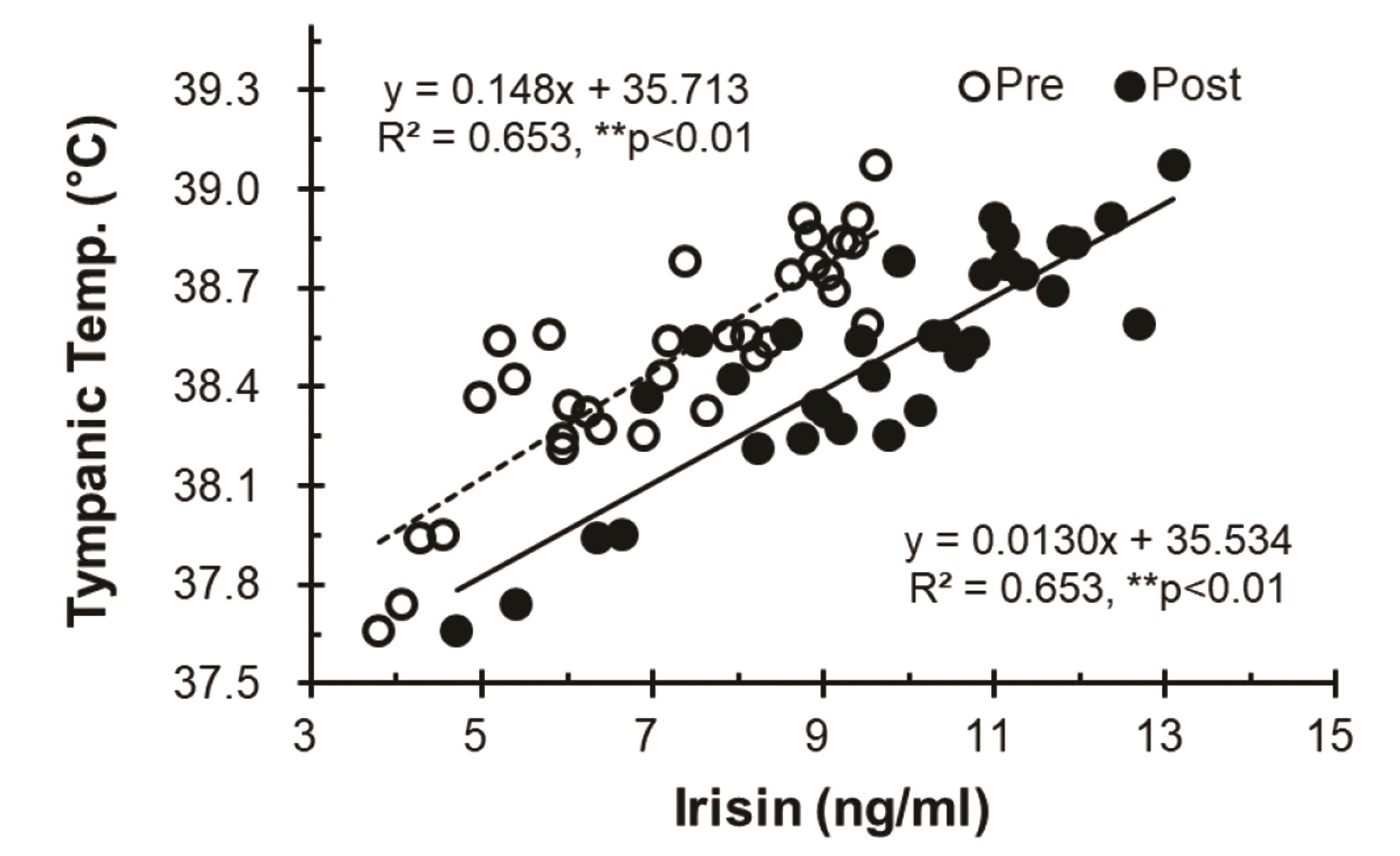
Fig. 6
Changes in adiponectin levels based on thermotherapy.
Values (n = 33) are mean ± SD. Statistically significant differences were found between Pre and Post, ***p < 0.001, and significant differences between TH-1 and TH-15, ##p < 0.01. TH, thermotherapy, immersing half-bath, 42 ± 0.5°C for 60 min (5-min break at 5-min intervals); TH-1, application of thermotherapy once; TH-15, application of thermotherapy repeated 15 times.
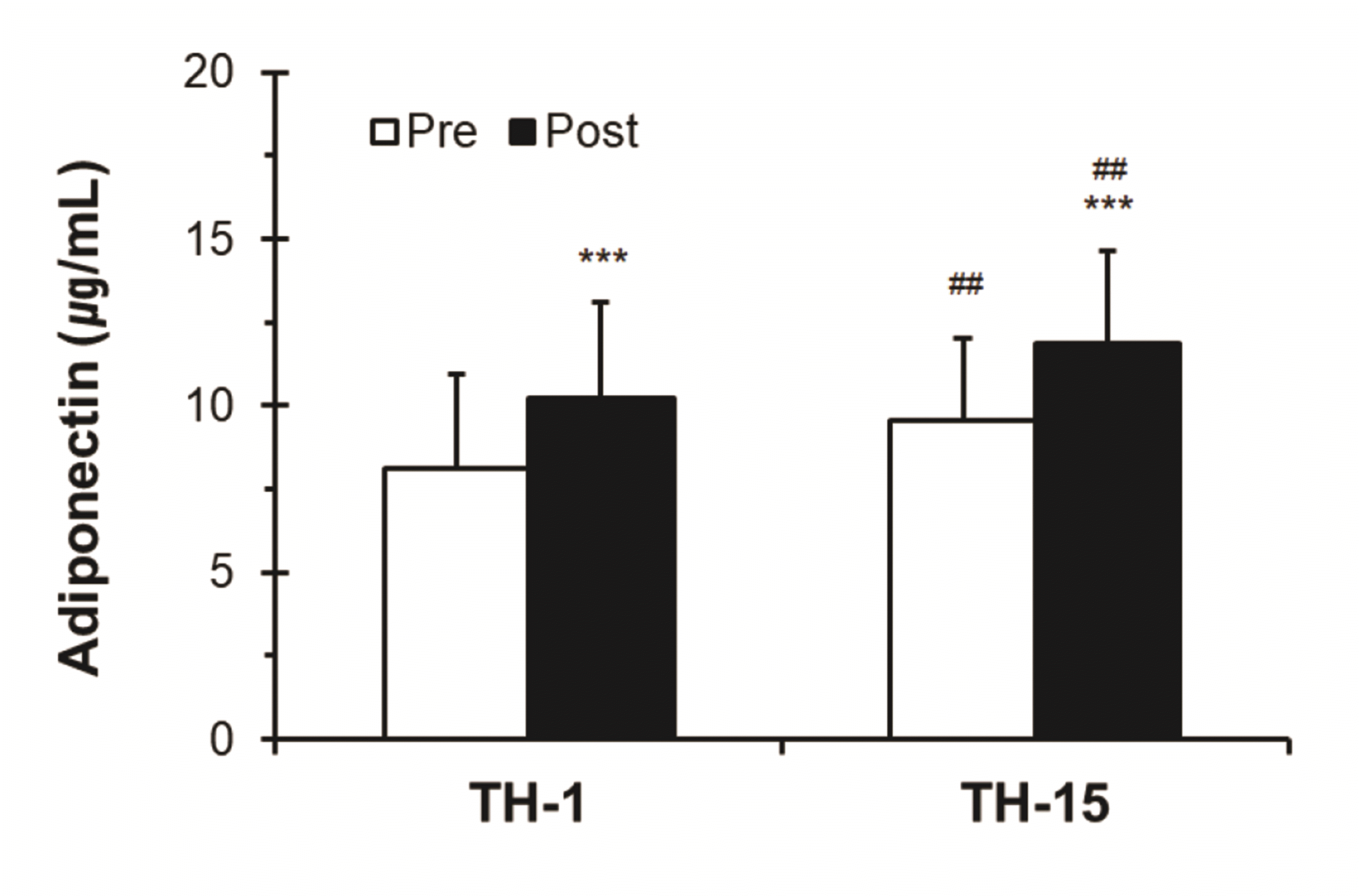
Fig. 7
Changes in the levels of free fatty acids (FFA) based on thermotherapy.
Values (n = 33) are mean ± SD. Statistically significant differences were found between Pre and Post, ***p < 0.001, and significant differences were found between TH-1 and TH-15, ##p < 0.01. TH, thermotherapy, immersing half-bath, 42 ± 0.5°C for 60 min (5-min break at 5-min intervals); TH-1, application of thermotherapy once; TH-15, application of thermotherapy repeated 15 times.
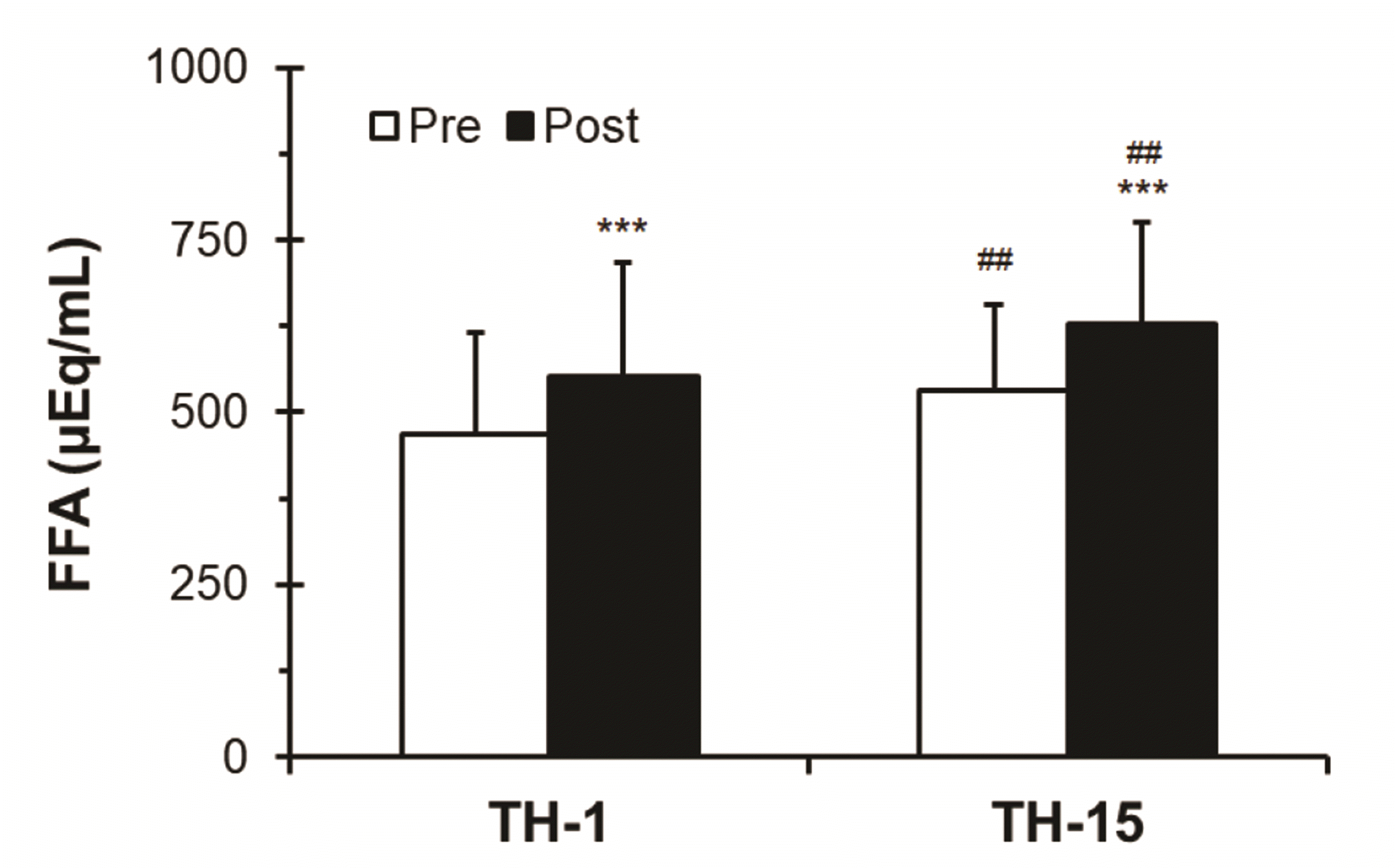
Fig. 8
Changes in the levels of whole body sweat loss volume based on thermotherapy.
Values (n = 33) are mean ± SD. Statistically significant differences between TH-1 and TH-15 (###p < 0.001). TH, thermotherapy, immersing half-bath, 42 ± 0.5°C for 60 min (5-min break at 5-min intervals); TH-1, application of thermotherapy once; TH-15, application of thermotherapy repeated 15 times.
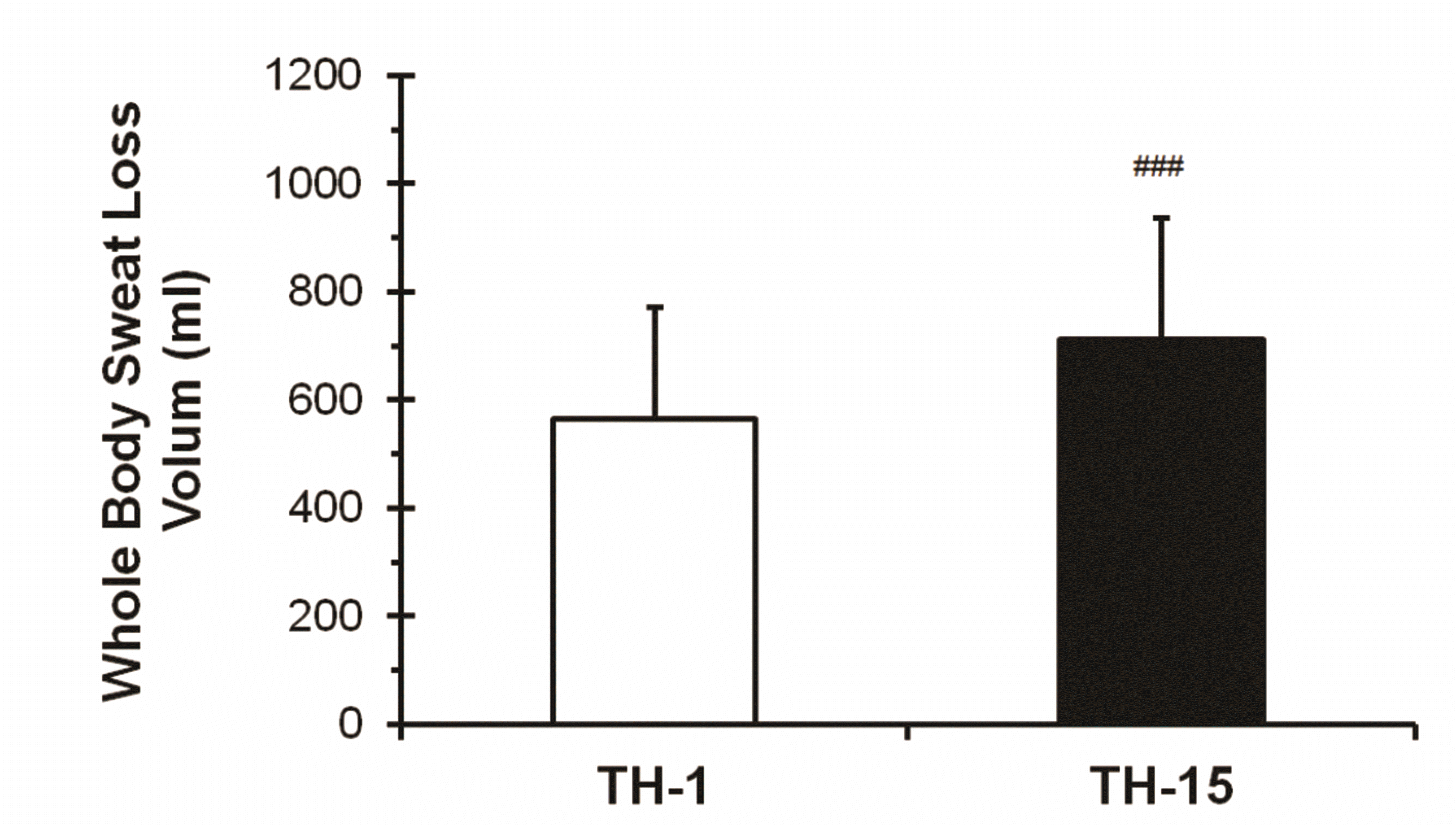
Table 1
Changes in obesity indices after thermotherapy
| Variable | TH-1 | TH-15 | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| BMI (kg/m2) | 27.19 ± 3.40 | 26.81 ± 3.05* | 25.84 ± 3.12# | 25.31 ± 3.17**,### |
| WC (cm) | 85.43 ± 8.12 | 84.27 ± 8.30** | 83.49 ± 7.25# | 82.14 ± 7.30***,### |
| % Body fat | 32.75 ± 4.06 | 32.29 ± 4.12* | 31.35 ± 3.51## | 30.67 ± 3.61***,### |
Values (n = 33) are means ± SD. TH, thermotherapy, half-bath, 42 ± 0.5°C for 60 min (5-min break at 5-min intervals); TH-1, application of thermotherapy once; TH-15, application of thermotherapy 15 times; BMI, body mass index (calculated from height and weight, BMI = weight (kg) / [height (m) × height (m)]; WC, waist circumference. Statistically significant differences were found between Pre and Post, *p < 0.05, **p < 0.01, ***p < 0.001, and significant differences between TH-1 and TH-15, #p < 0.05, ##p < 0.01, ###p < 0.001.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download