INTRODUCTION
METHODS
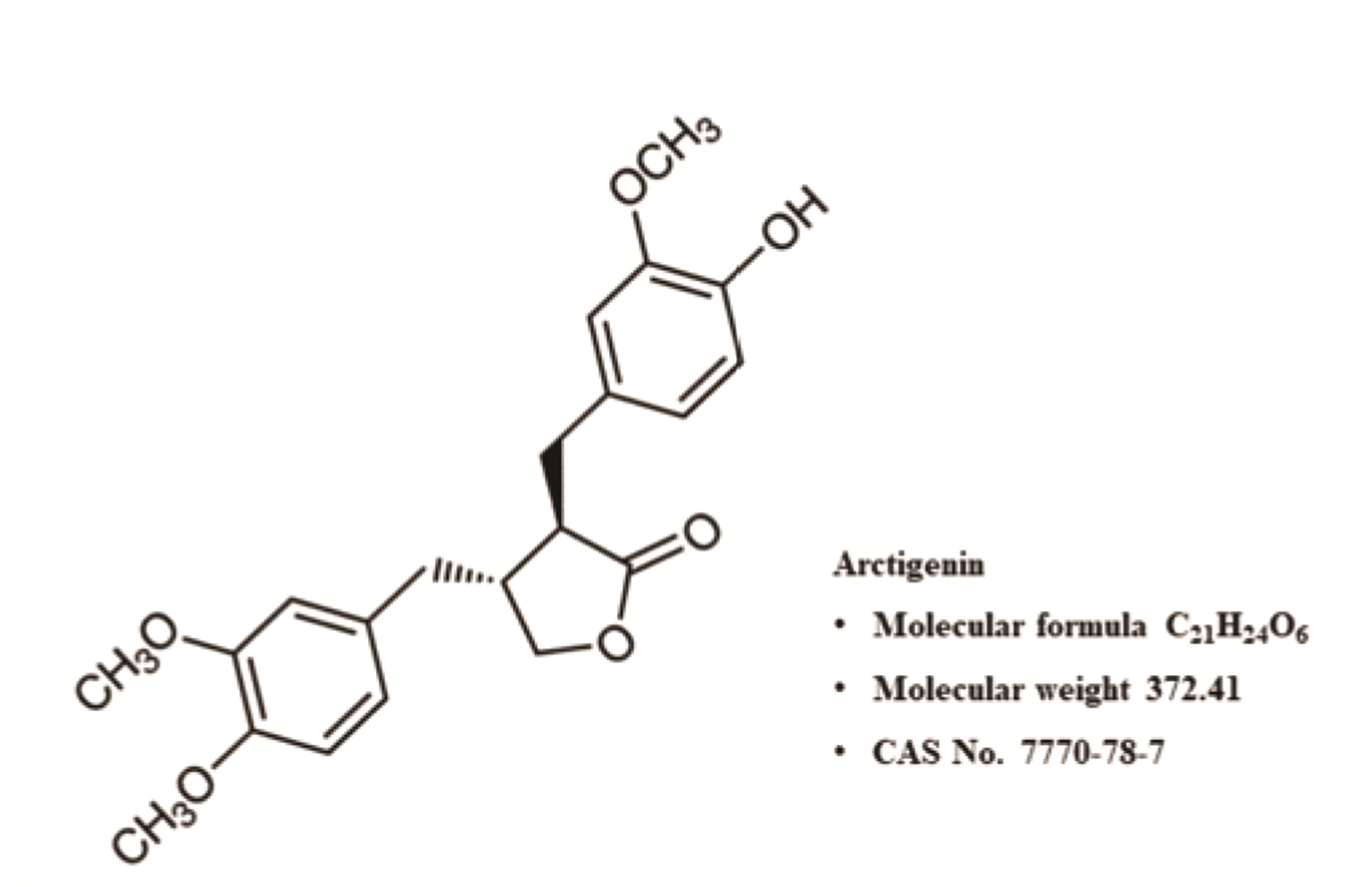
Reagents
Cell line and cell cultures
Cell viability test (MTT assay)
Live/Dead cell assay
DAPI staining
TUNEL assay
Caspase-3/-7 activity assay
Immunoblotting
Statistical analysis
RESULTS
Arctigenin has cytotoxic effects on FaDu human pharyngeal carcinoma cells
 | Fig. 2Effect of Arctigenin on cell viability in FaDu pharyngeal carcinoma cells.The effect of Arctigenin on the viability of L-929 cells (A) and FaDu cells (B) was accessed by the MTT assay. The percentage of cell viability was calculated as a ratio of A570nms of Arctigenin treated with cells and untreated control cells. Each data point represents the mean ± SEM of four experiments. MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide. **p < 0.01 vs. control and ***p < 0.001 vs. control (the control cells measured in the absence of Arctigenin).
|
 | Fig. 3Induction of cell death by Arctigenin.(A) L-929 cells and FaDu cell death by Arctigenin. The cells were treated with 0, 50, or 100 μM Arctigenin for 24 h. Arctigenin induced the death of FaDu cells in the dose-dependent manner. FaDu cells emitting green fluorescence are live cells stained by green calcein AM, whereas cells emitting red fluorescence are dead cells stained by ethidium homodimer-1. The arrows indicate dead cells stained red. Scale bar represents 100 µm. (B) Quantitative data of (A) were counted, and calculated as 100%. Each data point represents the mean ± SEM of four experiments. **p < 0.01 vs. control and ***p < 0.001 vs. control (the control cells measured in the absence of Arctigenin).
|
Arctigenin induces apoptotic cell death in FaDu pharyngeal carcinoma cells
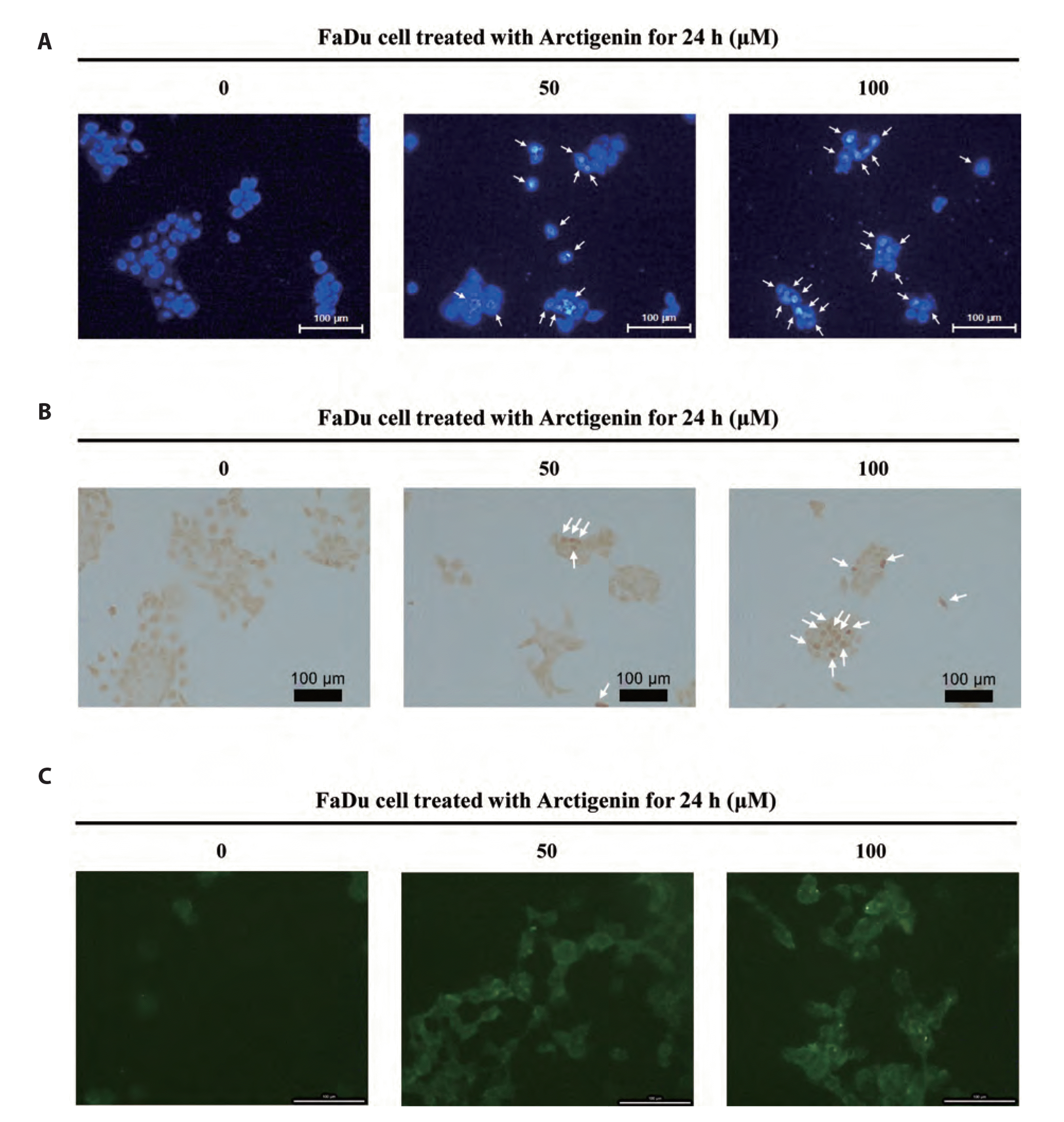 | Fig. 4Apoptotic phenomenon in FaDu pharyngeal carcinoma cells stimulated with Arctigenin.(A) Changes in nuclear morphology by Arctigenin. The cells were treated with 0, 50, 100 μM Arctigenin for 24 h. DAPI staining revealed that the number of FaDu cells with nuclear condensation was increased by Arctigenin. The arrows indicated the apoptotic cells with chromatic condensation characteristics. Scale bar represents 100 µm. (B) TUNEL assay revealed that the number of FaDu cells with apoptotic nuclei are stained dark brown was increased by Arctigenin. The arrows indicate apoptotic nuclei stained dark brown. Scale bar represents 100 µm. (C) Arctigenin induces the caspase-3/-7 activation in FaDu cells. The caspase-3/-7 intracellular activity assay was performed using PhiPhiLux-caspase-3/-7 substrate. Images were observed by fluorescence microscopy (Eclipse TE 2000; Nikon Instruments, Melville, NY, USA). Scale bar represents 100 µm.
|
Arctigenin-induced cell death is mediated extrinsic death receptor-dependent and intrinsic mitochondria-dependent apoptotic pathways
 | Fig. 5Arctigenin-induced FaDu pharyngeal carcinoma cell death is meditated by both intrinsic and extrinsic pathways.(A) Extrinsic death receptor-mediated apoptotic signaling pathway induced by Arctigenin. Arctigenin upregulated the expression level of the death receptor ligand Fas and subsequently activated the extrinsic death receptor-mediated apoptotic signaling pathway through the cleavage of caspase-8 in FaDu cells. (B) Intrinsic mitochondria-dependent apoptotic signaling pathway induced by Arctigenin. Arctigenin downregulated anti-apoptotic factors Bcl-2 and Bcl-xL associated with the intrinsic mitochondria-dependent apoptotic pathway and upregulated the mitochondria-dependent pro-apoptotic factors Bax and Bad in FaDu cells. (C) Extrinsic death receptor-mediated and intrinsic mitochondria-dependent apoptosis signaling pathways via the activation of caspase-3 and PARP induced by Arctigenin. Cleaved caspase-8 and cleaved caspase-9 induced the activation of caspase-3 and PARP in FaDu cells treated with Arctigenin.
|
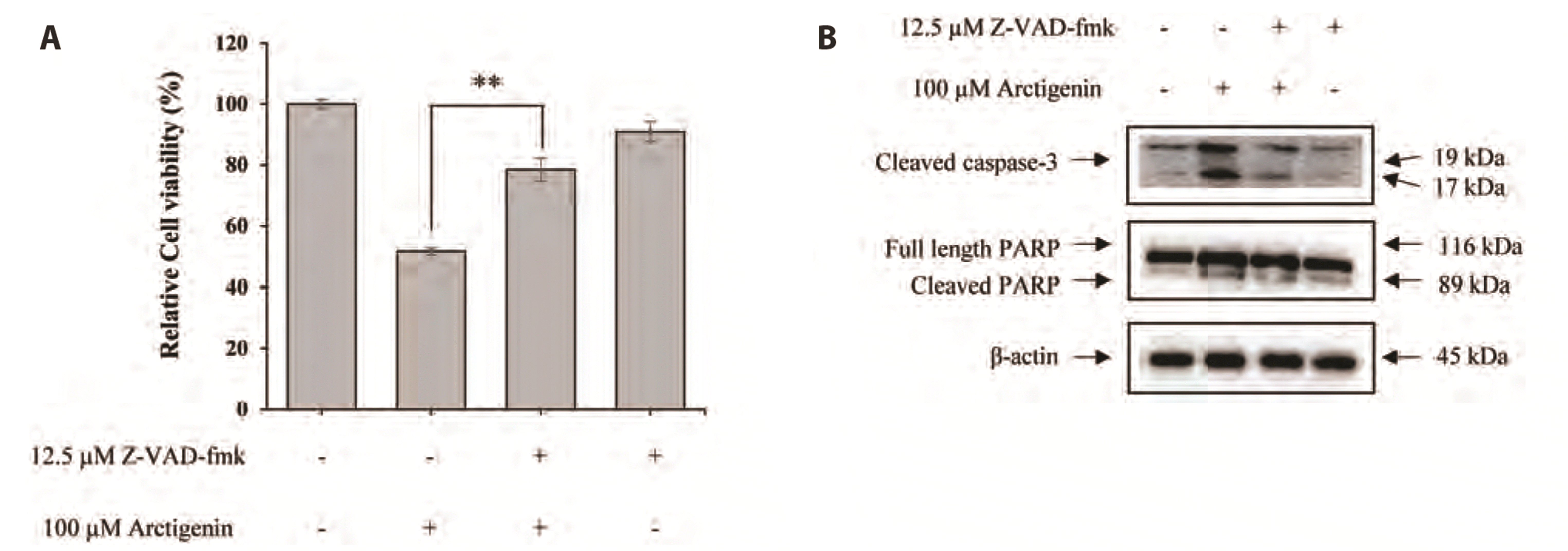 | Fig. 6Arctigenin-induced apoptosis in FaDu cells was mediated by caspase.(A) Z-VAD-fmk, a pan-caspase inhibitor, reversed Arctigenin induced cell death in FaDu cells. (B) Activation of caspase-3 and PARP in FaDu cells treated with Arctigenin was inhibited in the presence of Z-VAD-fmk. Each data point represents the mean ± SEM of four experiments (**p < 0.01).
|
Arctigenin-induced FaDu cell death is induced by ERK and p38 MAPK signaling pathways
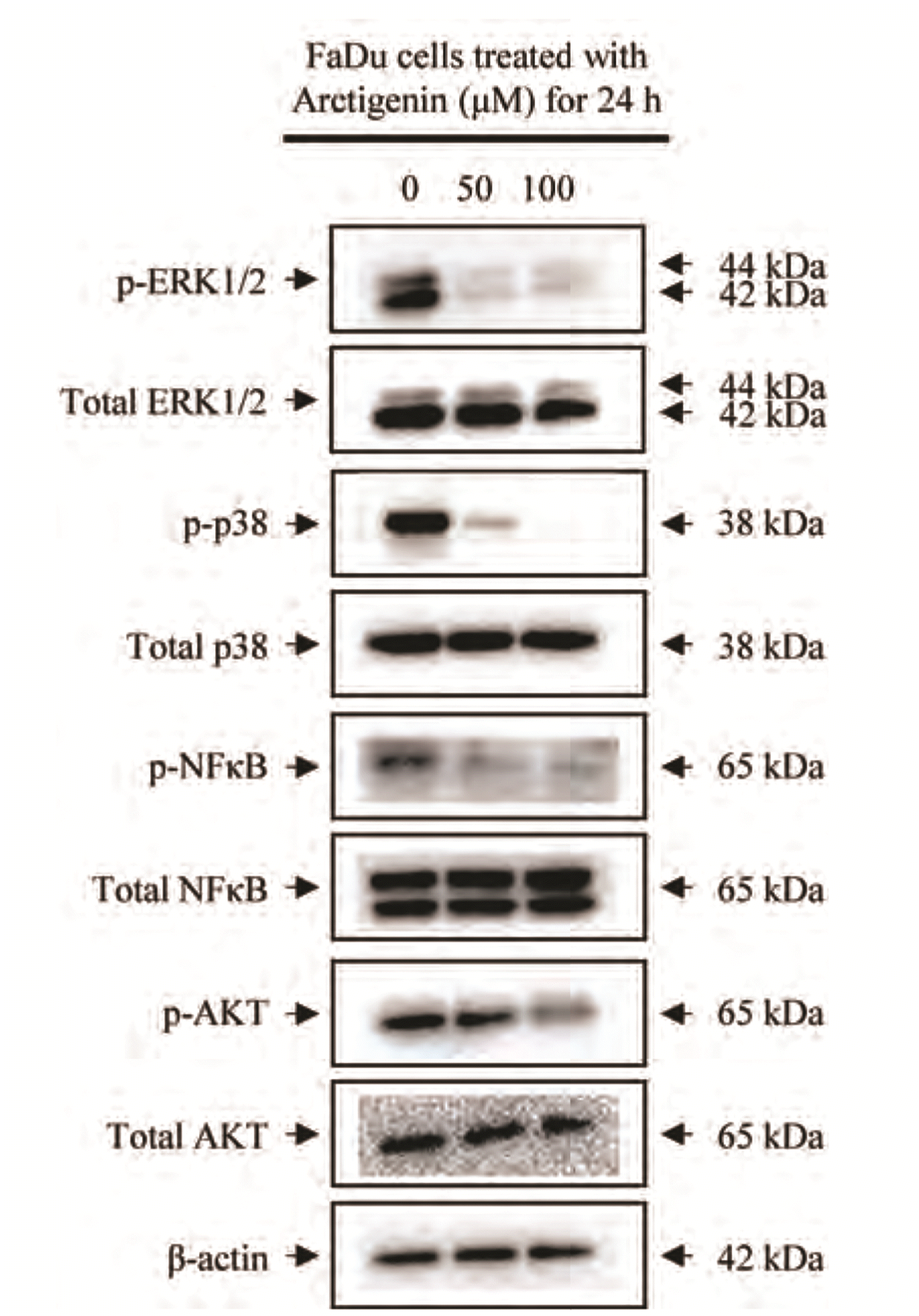 | Fig. 7Arctigenin reduced the phosphorylation of proteins in the MAPKs, NF-κB, and AKT pathways in FaDu pharyngeal cells.FaDu cells were treated with 0, 50, and 100 μM Arctigenin for 24 h. Thereafter, immunoblotting using specific antibodies against extracellular signal-regulated kinase 1/2 (ERK1/2), p38, NFκB, and AKT was performed to verify potential cellular signaling pathways associated with Arctigenin-induced apoptosis.
|




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download