INTRODUCTION
HAIR FOLLICLE DEVELOPMENT AND HAIR CYCLE
THE IMPLICATION OF COX IN HAIR GROWTH
PROSTAGLANDINS AND PROSTAGLANDIN RECEPTORS IN HAIR GROWTH
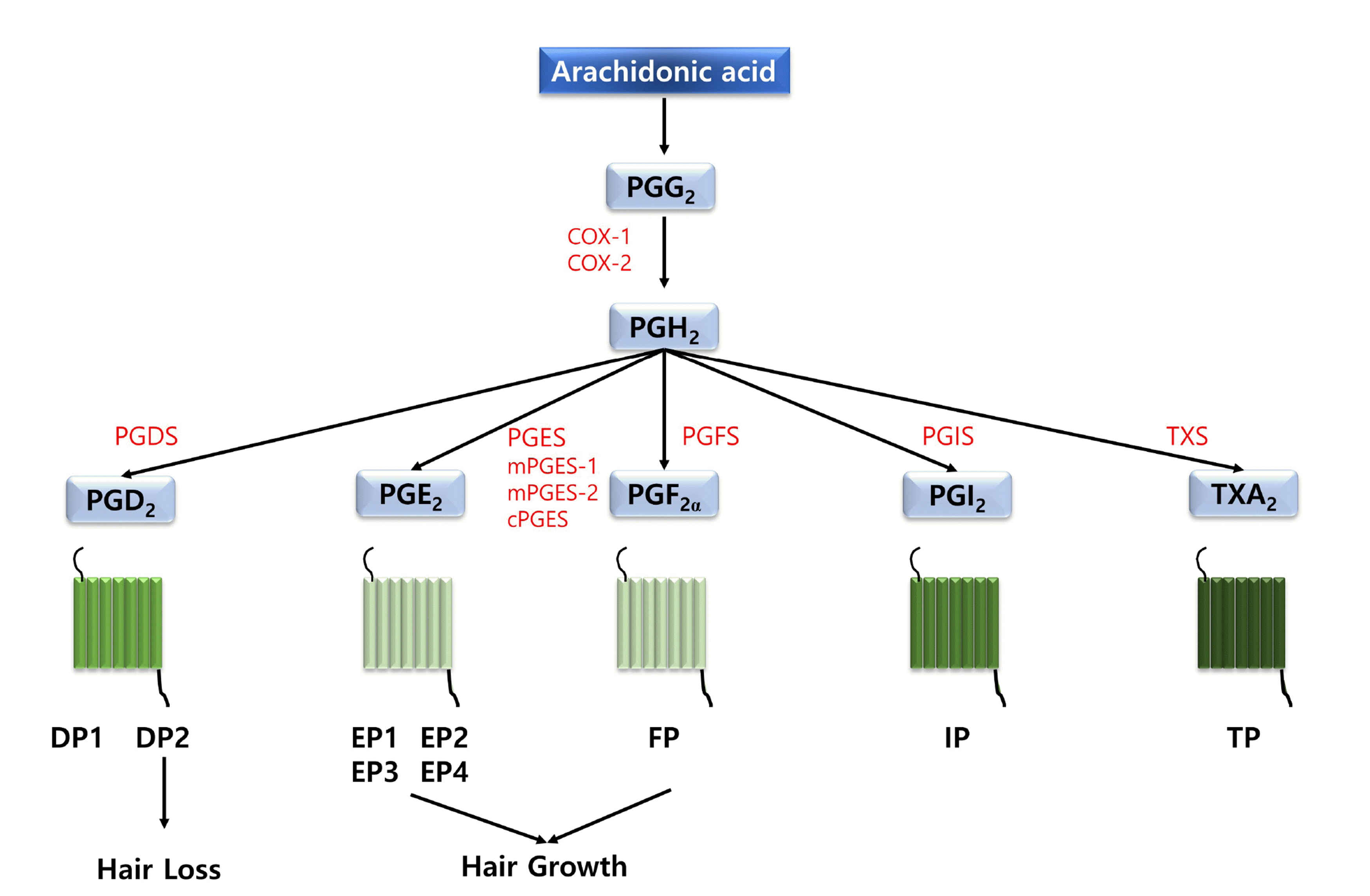 | Fig. 1Diagram of prostanoids and their biological effects on hair follicles through stimulating different type of G protein-coupled receptors.Prostanoids are classified into five types, namely thromboxane A2 (TXA2), prostaglandin D2 (PGD2), prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), and prostaglandin I2 (PGI2). PGD2 binds to prostaglandin D2 receptors (DP1, DP2), whereas PGE2 binds to prostaglandin E2 receptors (EP1, EP2, EP3, EP4). PGF2α binds to the prostaglandin F2α receptor (FP). PGI2 binds to the prostaglandin I2 receptor (IP). TXA2 binds to the thromboxane A2 receptor (TP). Human hair follicles remarkably expressed microsomal PGES (mPGES)-1, mPGES-2, and cytosolic PGES (cPGES), which converts PGH2 to PGE2. These human hair follicles also significantly expressed AKR1C3/PGFS, which catalyzes PGH2 to PGF2α. In addition, cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2), prostaglandin G2 (PGG2), prostaglandin H2 (PGH2), PGD2 synthase (PGDS), PGE2 synthase (PGES), PGF2α synthase (PGFS), PGI2 synthase (PGIS) are marked.
|
THE INHIBITORY ROLES OF PROSTAGLANDIN (PGD2) IN HAIR GROWTH
Table 1
|
Compound name (type) |
Subjects | Working concentration & condition | Positive effects or molecular targets | References |
|---|---|---|---|---|
| Cetirizine(Anti-histamine, PGD2 inhibitor) | 60 patients with AGA | 1% cetirizine-treated group for 6 months | ↑ Hair growth | [37] |
| 40 males with AGA | 1% cetirizine solution for 16 weeks | ↑ Total & vellus hair density | [38] | |
| ↑ Anagen phase ratio | ||||
| 67 males with AGA | Topical 1% cetirizine per daily | ↑ Total hair density | [61] | |
| ↑ Terminal hair density | ||||
| Setipiprant(DP2 antagonist) | 83 males with AGA | Phase 2a clinical trial 500 mg twice a day for 24 weeks | Safety and tolerability | [39] |
| Not significant efficacy | ||||
| Levocetirizinehydrochloride (Anti-histamine, PGD2 inhibitor) | Human dermal papilla cells | 1, 10, 100, 1,000, and 10,000 ng/ml for 24 h | ↓ PDG2, PTGDS, DP2 | [40] |
| ↑ PGF2α, | ||||
| ↑ pAKT, pGSK3β | ||||
| Latanoprost(PGF2α analog) | 43 patients with glaucoma | Unilateral topical 0.1% latanoprost administration for 10 weeks | ↑ Hypertrichosis, | [41] |
| ↑ Pirgmentation | ||||
| 54 males with alopecia areta | Latanoprost 0.005% (50 μg/ml) ophthalmic solution for 3 months to 2 year | ↑ Hair regrowth | [42] | |
| 16 males with AGA | 0.1% latanoprost in males with mild AGA for 24 weeks | ↑ Hair density, hair growth | [45] | |
| ↑ Hair diameter | ||||
| 30 males with alopecia areta | Topical 0.005% latanoprost for 12 weeks | ↑ Hair density | [46] | |
| ↑ Hair regrowth | ||||
| Bimatoprost(PGF2α analog) | 585 subjects | 0.03% ophthalmic bimatoprost solution for at least 12 months | ↑ Eyelashes regrowth | [50] |
| 30 patients | 0.03% bimatoprost ophthalmic solution for 1 year | ↑ Eyebrow thickness | [52] | |
| 238 patients with idiopathic hypotrichosis | 0.03% bimatoprost ophthalmic solution for 1 year | ↑ Eyelash regrowth | [53] | |
| 36 patients with alopecia areata 40 subjects | 0.03% bimatoprost for 6 months | ↑ Eyelash regrowth | [54] | |
| Human dermal papilla cells | 0.03% bimatoprost | ↑ Eyelash regrowth in healthy adolescents | [58] | |
| AGA mouse model(MaleC57BL/6J mice) | 5 μM bimatoprost in solvent mixture system | ↑ Cell proliferation | ||
| 0.3%–5% bitmatoprost formulation for 14 days | ↑ Hair regrowth | [59] | ||
| ↑ Hair weight | ||||
| ↑ Diameter & number of Hair follicles |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download