INTRODUCTION
Hypopharyngeal squamous cell carcinoma constitutes 5%–15% of head and neck squamous cell carcinoma (HNSCC) and is the sixth most common cancer in the world. Its prevalence has markedly increased in developing countries, accounting for approximately 20%–35% of all the HNSCC cases reported globally [
1,
2]. In particular, patients with hypopharyngeal cancer have the worst prognosis among the patients with advanced head and neck cancer and poor five-year survival rates. For example, the five-year survival rate of patients with hypopharynx cancer at stage IV in Taiwan is only 24.8% [
3]. Conventional treatment for hypopharyngeal squamous cell carcinoma is a combination of surgery, radiotherapy and chemotherapy, in which concomitant chemoradiotherapy promotes locoregional tumor control and increases survival rates compared to radiotherapy alone.
Valdecoxib (Val) is a nonsteroidal anti-inflammatory drug, which was approved by United States Food and Drug Administration (FDA) for the treatment of inflammatory diseases; however, the drug was withdrawn from the US market in 2005 due to the reported side effects including increased risk of cardiovascular adverse events and serious skin reactions. Recently, antitumor effects of Val and other COX-2 inhibitors (e.g., celecoxib) have been reported as the drugs induced cell proliferation arrest and apoptosis in nasopharyngeal carcinoma cells, and promoted cell lysis in lung cancer cells [
4,
5]. In addition, combining celecoxib with other therapeutics, such as capecitabine or cyclophosphamide, improved the clinical benefits while reducing the toxicity of monotherapy in metastatic breast cancer [
6]. Val also exerted the potential effects of increasing lipid composition, order, and dynamics in colon cancer [
7]. Despite these promising reports, the antineoplastic effect and molecular mechanism of action of Val in HNSCC are not well understood. Therefore, we investigated the antitumor activity of Val in FaDu hypopharyngeal squamous carcinoma cells and attempted to elucidate its cellular mechanism of action. Here, we report that Val has antitumor effects on cell cycle regulatory proteins, caspases, integrins, mitogen-activated protein kinase (MAPK) pathway, and PI3K/AKT/mTOR pathway, which lead to the suppression of cell proliferation and mobility, as well as the induction of apoptosis.
Go to :

METHODS
Antibodies and reagents
Val (purity ≥ 98.0%), dimethylsulfoxide (DMSO), diphenyleneiodonium (DAPI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Minimum essential medium Eagle (MEM), fetal bovine serum (FBS), trypsin-ethylenediaminetetraacetic acid (EDTA), and antibiotic-antimycotic solution were obtained from Welgene (Daegu, Korea). Primary antibodies against cyclins (A, B1, and E), Cdk2, Cdk4, Cdk7, CDC25C, precursor caspase-3, caspase-9, p-AKT, p-mTOR, MMP1, MMP2, MMP9, fibronectin, VCAM-1, and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The cleaved PARP antibody was purchased from Abcam (Cambridge, MA, USA). Cyclin D1, cleaved caspase-3, p-p42/p44 ERK1/2, p-p38 MAPK1/2, p-JNK, p-H2AX, p-CHK2, p-PI3K, PI3K p85, and integrins were obtained from Cell Signaling Technology (Danvers, MA, USA).
Cell culture
FaDu hypopharyngeal squamous carcinoma cells were grown in MEM, supplemented with 10% FBS and 100 units/ml penicillin/streptomycin at 37°C in a humidified atmosphere with 5% CO2. The cells (2 × 105 cells/ml) were incubated for 24 h before being treated with various concentrations of Val (0, 25, 50, and 75 μM).
Cell viability assay
Cell viability was determined using a substrate consisting of MTT. Briefly, the cells were seeded at a density of 4 × 104 cells/well in 96-well plates for 24 h and then treated with various concentrations of Val. After 48 h, the media was removed, and 50 μl of MTT solution (0.5 mg/ml) was added to each well and incubated for 4 h at 37°C. Upon the formation of violet crystals, the MTT solution was removed and 100 μl of DMSO was added to dissolve the formazan crystals. The optical density was measured at a wavelength of 540 nm using a Multiskan FC microplate photometer (Thermo Fisher, Waltham, MA, USA).
Wound healing assay
FaDu cells (2 × 105 cells/well) were seeded onto coverslips in a 24-well plate to reach 80% confluence. Using pipette tips, a scratch was made in the middle of the coverslips. The cells were treated with Val (0, 25, 50, and 75 μM) for 48 h in an incubator. The images of the wounds were captured using a Zeiss AxioscopeA1 microscope (Microscope World, Carlsbad, CA, USA), and the width of wound closure in the captured images was determined using an iSolution software (IMT i-solution Inc., Riverton, UT, USA).
Transwell assay
Transwell assays were performed using 24-well Transwell chambers (pore size: 8 μm; EMD Millipore, Billerica, MA, USA). Briefly, after treatment with Val for 48 h, cells were collected and suspended in serum-free MEM. For the migration assay, 1 × 10⁴ cells in 200 μl of serum-free media were seeded onto the upper chambers. For the invasion assay, the membranes were coated with 100 μl of serum-free MEM containing extracellular matrix (2 μg/ml) for 2 h at 37°C and 100 μl of cell suspension was added. In both assays, the lower chambers were filled with 750 μl of basal MEM and incubated at 37°C. After 24 h, cells on the bottom were washed with PBS and fixed with 4% paraformaldehyde. Cells in the upper compartments were gently removed using cotton swabs, and the cells on the lower side of inserts were stained with 2% crystal violet for 15 min at room temperature. The membranes were then washed with distilled water to remove the stained solution. Images of cells were captured using a Zeiss AxioscopeA1 microscope (Microscope World). These cells were dissolved in 10% cetylpyridinium chloride solution, and the optical density was measured at 590 nm using a Multiskan FC microplate photometer (Thermo Fisher).
Western blotting
FaDu cells (2 × 105 cells/ml) grown in 60 cm plates for 24 h were treated with Val for 48 h. Thereafter, proteins were extracted using lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM PMSF) on ice for 30 min. Protein concentration was determined using the Bradford method (Thermo Scientific, Rockford, IL, USA). Total proteins from cell lysates were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 12% resolving gel) and subsequently transferred to nitrocellulose membranes using the Bio-Rad wet blot system (Bio Rad Laboratories, Hercules, CA, USA). After blocking with 5% skimmed milk for 1 h, the membranes were incubated with primary antibodies at 4°C overnight, followed by incubation with HRP-conjugated secondary antibodies for 2 h. Finally, proteins were detected using chemiluminescent reagents, and images were captured using the Kodak Digital Science Image Station (Bruker BioSpin, Billerica, MA, USA).
Immunofluorescence imaging
FaDu cells (2 × 105 cells/well) seeded onto coverslips were grown in 24-well plates for 24 h and then treated with Val (0, 25, 50, and 75 μM) for 48 h. After washing with PBS, cells were fixed with 4% paraformaldehyde for 15 min at room temperature and then again washed with PBS. The fixed cells were blocked and permeabilized with washing solution (PBS containing 0.1% Triton X-100 and 0.1% goat serum) for 1 h at room temperature. Thereafter, the cells were probed with Cdk1 and p-H2AX antibodies at 4°C overnight. Once washed with washing solution, the cells were incubated with goat anti-mouse Alexa Fluor 488-labeled IgG (1:1,000) (to detect Cdk1), goat anti-rabbit Alexa Fluor 495-labeled IgG (1:1,000) (to detect p-H2AX), and DAPI for 2 h at room temperature. Finally, coverslips were mounted onto microscopic slides and cell images were captured using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Go to :

DISCUSSION
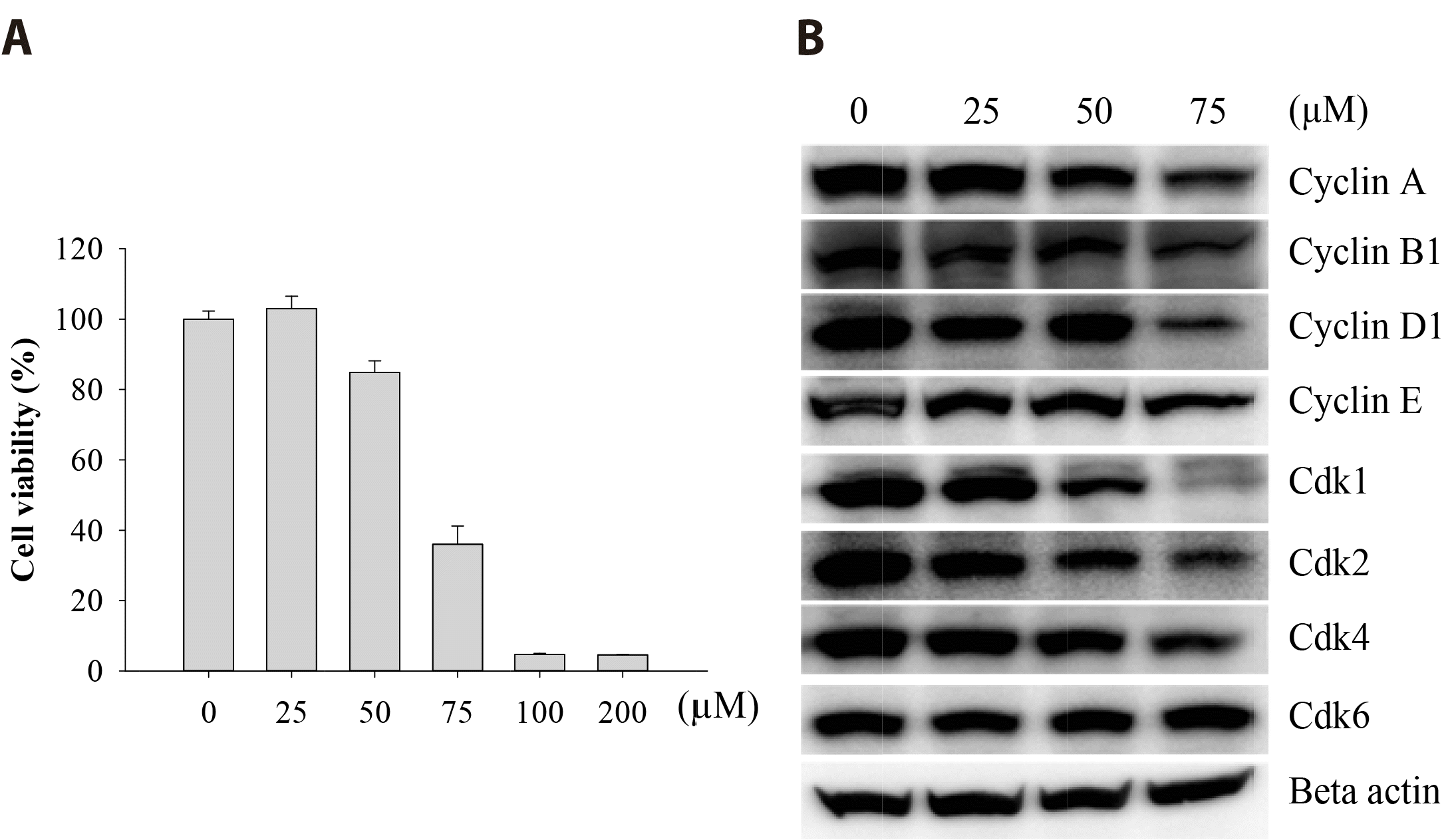
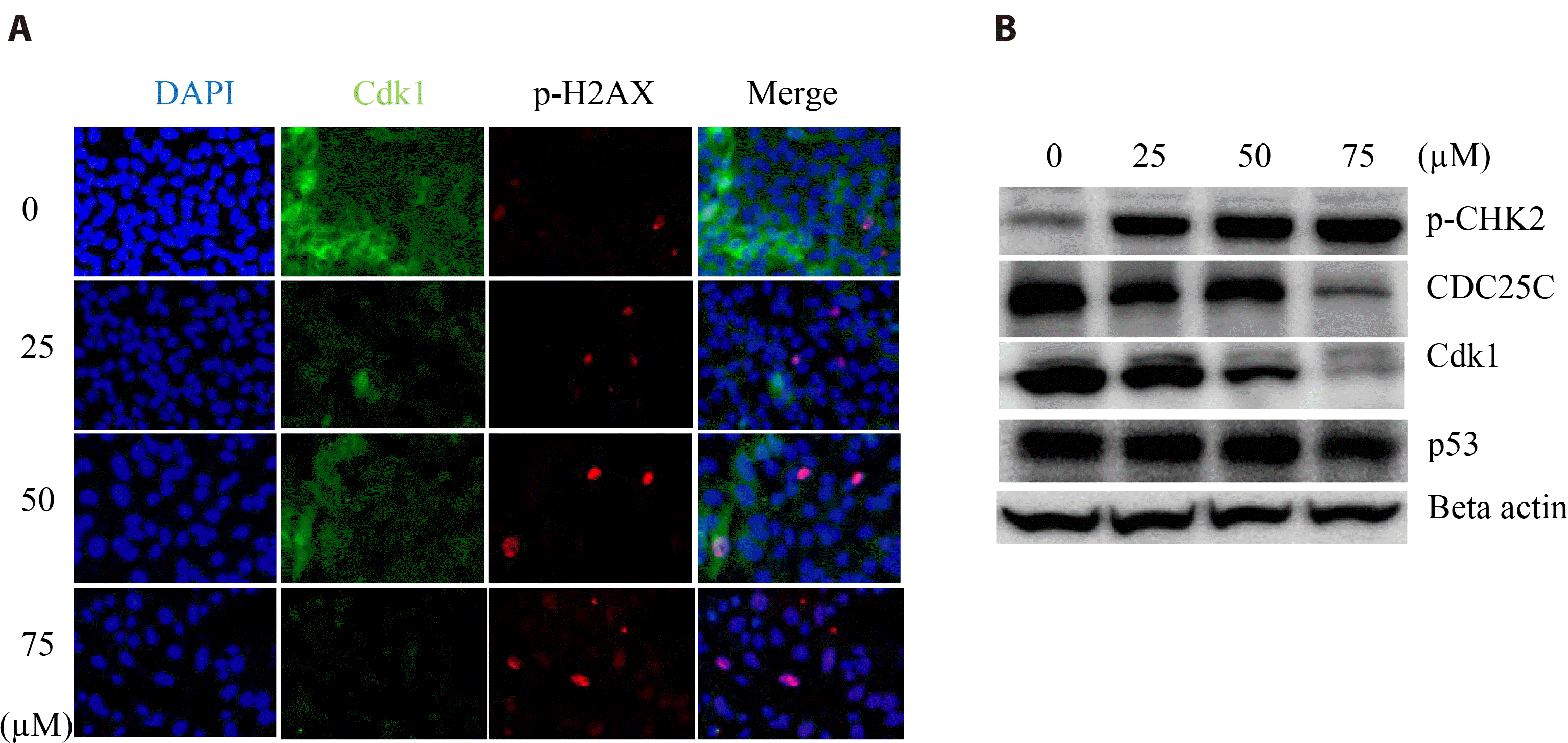
In this study, we investigated the anti-cancer activity of Val in hypopharyngeal squamous cell carcinoma using FaDu cells. Val inhibited cell proliferation in a dose-dependent manner and induced apoptosis through regulation of the CHK2/CDC25C/Cdk1 pathway. Various cell cycle regulatory proteins such as cyclins and Cdks were downregulated, including Cdk1, an archetypical kinase that plays an essential role in driving cells through the G2/M phase transition [
8]. The cyclin B1/Cdk1 complex translocates into the nucleus and activates a range of target proteins, which assist in transitioning of cell from interphase to mitotic phase [
9]. After Val treatment, the expression of both Cdk1 and cyclin B1 was significantly suppressed (
Fig. 1), and DNA damage was followed by increased expression of p-H2AX in the nucleus (
Fig. 5). The expression of CHK2, in response to DNA damage, negatively controls Cdk1 through CDC25 or p53 to repair the damaged DNA before entering mitosis. In this study, excessive augmentation of activated p-CHK2 under Val treatment and the corresponding decrease in CDC25C and Cdk1 expressions, which did not affect p53 expression, supported the hypothesis that Val suppressed FaDu cell proliferation through CHK2/CDC25C/Cdk1 signaling and that cell cycle arrest was p53-independent (
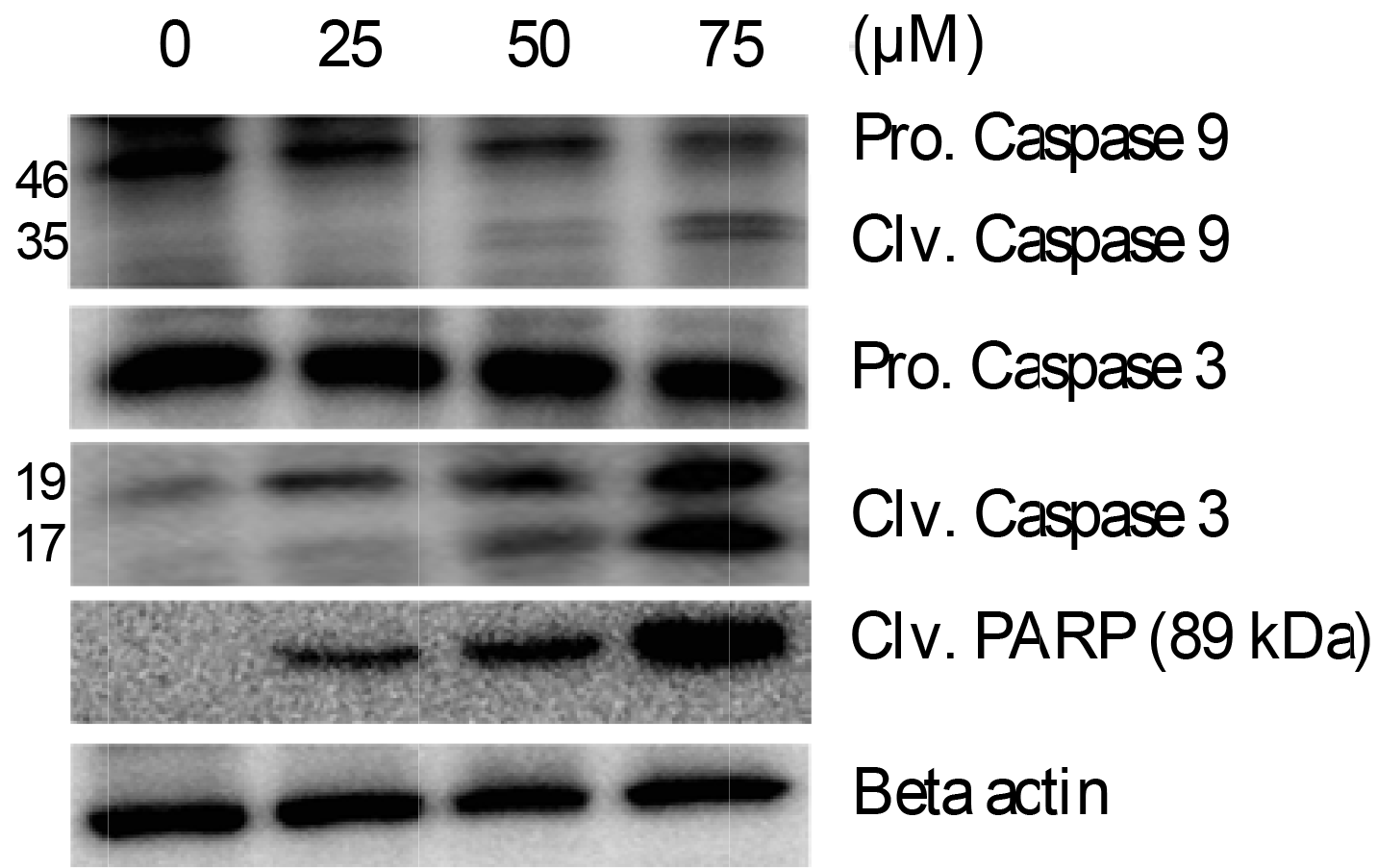
Fig. 5). The activation of CHK2 might have caused the phosphorylation and degradation of CDC25C, thus preventing the activation of downstream Cdk1 and cyclin B1 for the G2/M phase transition. Furthermore, Val-induced superabundant DNA damage outweighed the repair capacity of the cells, leading to programmed cell death through the activation of caspases (cleaved caspases-3 and -9) (
Fig. 4).
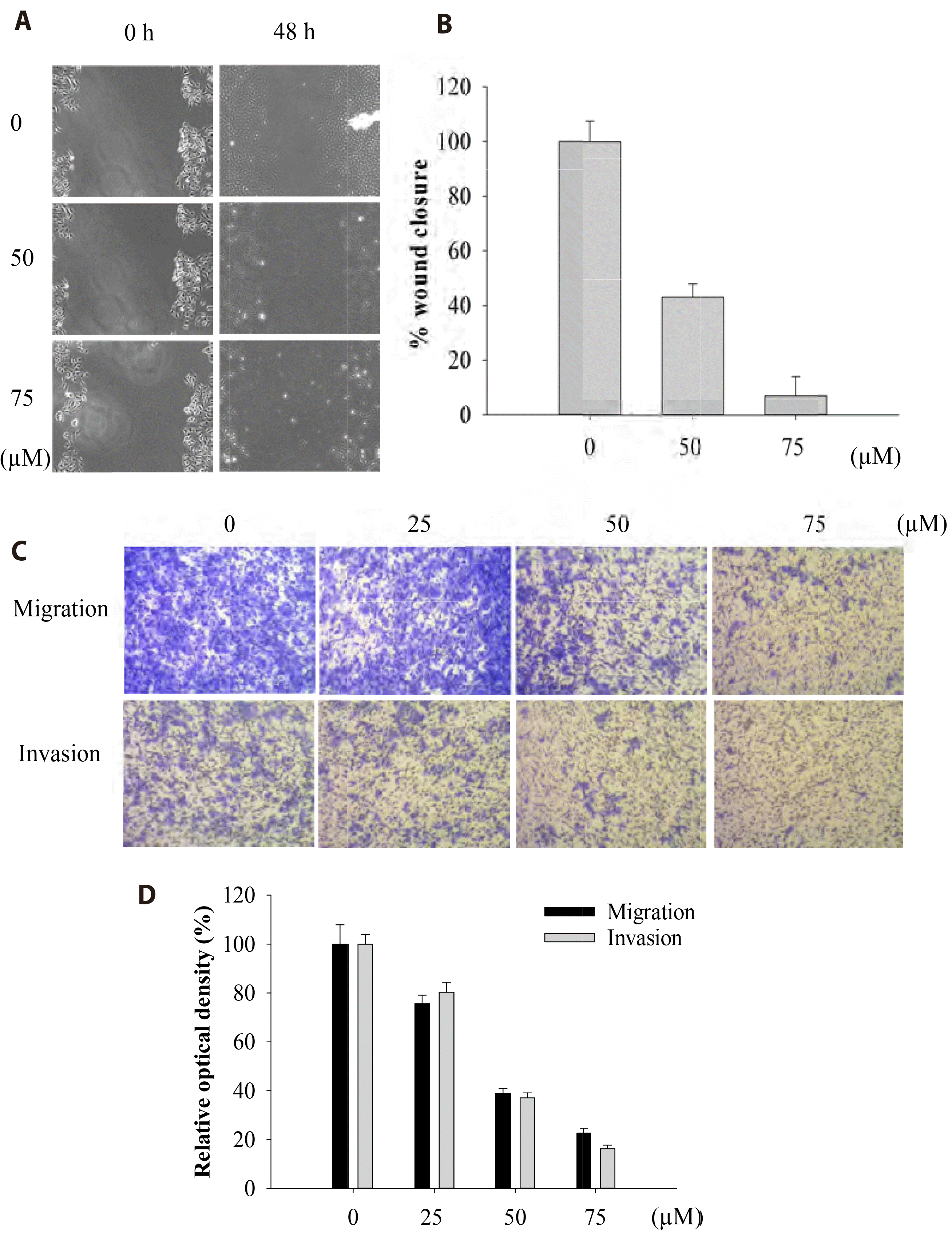
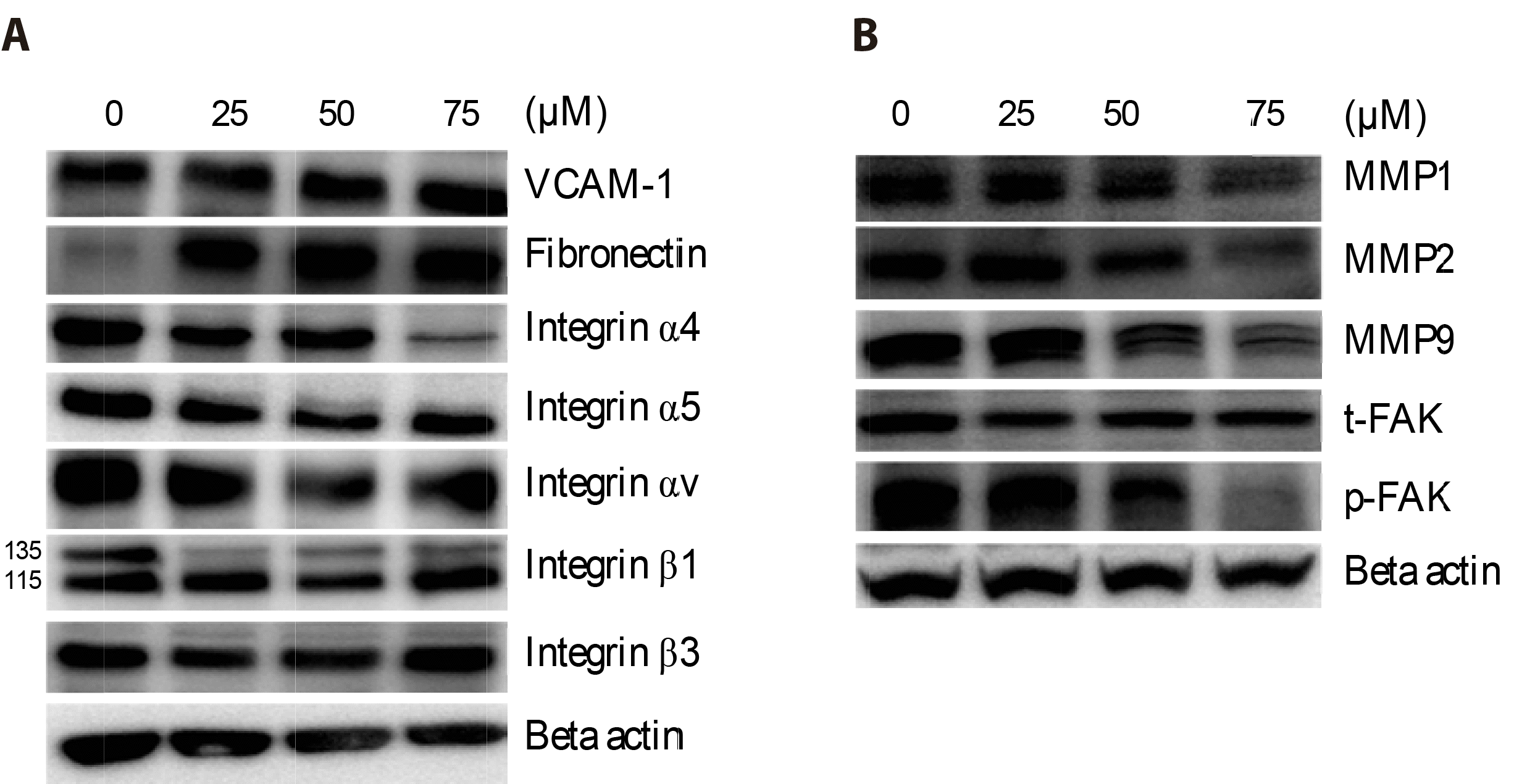
Val effectively inhibited tumor cell migration and invasion by downregulating the ITGA4/FAK pathway (
Fig. 2). Integrins are cell adhesion receptors that play important roles in cell signaling between the internal cytoskeletal network and extracellular environment [
10]. Among the integrins examined, ITGA4 was the most significantly suppressed by Val, especially at 75 μM concentration, while other integrins were moderately inhibited or unchanged. The relationship between ITGA4 and tumor aggressiveness has been reported for various types of cancers [
11]. Elevated expression of ITGA4 using specific substrates increases cell mobility in neuroblastoma, and an association between ITGA4 levels and tumor aggressiveness has been found in clinical cases [
12]. In addition, suppression of integrin α4β1 upregulates the efficacy of chemotherapy in ovarian cancer [
13]. In gastrointestinal stromal tumors, ITGA4 expression is associated with adverse prognostic features and survival rates [
14]. Notably, in our study, the expression of fibronectin and VCAM-1, extracellular matrix ligands that bind and regulate integrin signaling pathways, was considerably increased under Val treatment. This might be due to the compensatory action of FaDu cells to make effective use of the reduced expression of ITGA4 receptors under Val treatment. At high doses of Val, severe loss of ITAG4 receptors might have disrupted cell-cell and cell-extracellular matrix adhesion, leading to a subsequent decrease in downstream p-FAK (
Fig. 3). In addition, decreased expression of matrix metalloproteinases (MMP1, MMP2, and MMP9) following Val treatment suppressed cell mobility and restricted tumor growth and invasion.
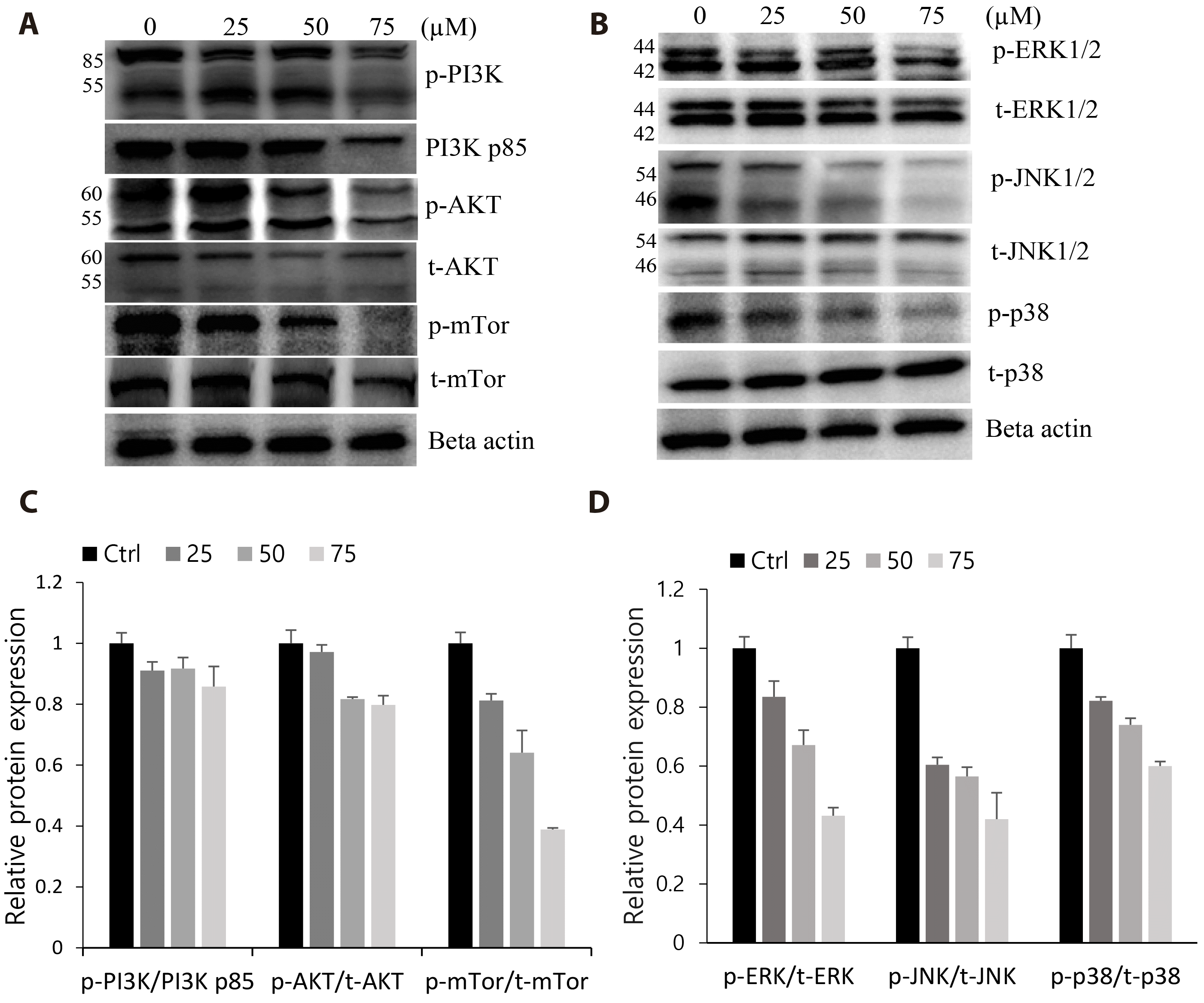
Val downregulated the key regulators of the PI3K/AKT/mTOR and MAPK cascades, supporting the suppression of proliferation, mobility, and apoptosis induction. The PI3K/AKT/mTOR pathway is over-activated in approximately 60% of HNSCC cases, and a crosstalk between the PI3K/AKT/mTOR and MAPK pathways has also reported [
15]. Thus, inhibiting a pivotal intracellular cascade or cotargeting both pathways could be crucial for HNSCC treatment [
16], as well as other cancers [
17,
18]. In conclusion, we confirmed that Val has antitumor effects on hypopharyngeal squamous cell carcinoma by suppressing cell proliferation and mobility, and inducing apoptosis. Our results support that Val could be a potential candidate for treating hypopharyngeal squamous cell carcinoma.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download