Abstract
Objectives
This study aimed to analyze the clinicopathological prognostic factors affecting the survival of patients with oral squamous cell carcinoma (OSCC).
Materials and Methods
A retrospective study was conducted on patients with OSCC who received treatment at the Oral Oncology Clinic of the National Cancer Center (NCC) from June 2001 to December 2020. The patients’ sex, age, primary site, T stage, node metastasis, TNM staging, perineural invasion (PNI), lymphovascular invasion (LVI), differentiation, surgical resection margin, smoking, and drinking habits were investigated to analyze risk factors. For the univariate analysis, a Kaplan–Meier survival analysis and log-rank test were used. Additionally, for the multivariable analysis, a Cox proportional hazard model analysis was used. For both analyses, statistical significance was considered when P<0.05.
Results
During the investigation period, 407 patients were received surgical treatment at the NCC. Their overall survival rate (OS) for five years was 70.7%, and the disease-free survival rate (DFS) was 60.6%. The multivariable analysis revealed that node metastasis, PNI, and differentiation were significantly associated with poor OS. For DFS, PNI and differentiation were associated with poor survival rates.
Oral cancer accounts for approximately 90% of oral squamous cell carcinoma (OSCC) and occurs anywhere in the oral cavity, such as the tongue, buccal mucosa, and gingiva. Approximately 377,000 new cases of oral cancer and 177,000 deaths occur annually worldwide1,2. However, patients with oral cancer have a relatively similar or poorer prognosis than those with other cancers, even though it occurs in a relatively easy-to-detect organ. Between 2015 and 2019, the five-year observed survival rate for all cancers in Korea was 65.6%. During the same period, the five-year observed survival rate for lip, oral, and pharyngeal cancers (C00-C14, ICD-10) was 64.3%3. The survival rate of cancers in the oral cavity is 48%-70%4,5. There are several prognostic factors after cancer therapy; however, the most widely used prognostic factor for patients with oral cancer is TNM staging according to the American Joint Committee on Cancer (AJCC). This prognostic factor relies on tumor size, metastasis to adjacent lymph nodes, and remote metastasis to other organs6. However, even if a cancer is classified at the same stage after treatment, post-treatment prognosis can vary for each patient. Therefore, other risk factors for prognosis should also be considered for these patients7. In this study, we retrospectively analyzed patients with OSCC who underwent surgical treatment at the Oral Oncology Clinic of the National Cancer Center (NCC) over a 20-year period between 2001 and 2020 to investigate the relative survival rate and risk factors affecting their survival.
A retrospective study was conducted on patients diagnosed with OSCC who underwent surgical treatment with or without adjuvant radiotherapy (RT) or concurrent chemoradiotherapy (CCRT) at the Oral Oncology Clinic of the NCC in South Korea between June 2001 and December 2020. This study was reviewed and approved by the Institutional Review Board (IRB) of the NCC (IRB No. NCC2022-0214). Surgery was perfomed with wide excision of the primary site with or without neck dissection. Patients with clinically single node metastasis or negative nodal disease, where there is high risk of occult metastasis, underwent selective neck dissection, whereas patients with multiple node metastasis underwent modified radical neck dissection. The patients’ clinicopathological data (sex, age, primary site, T stage, node metastasis, TNM stage, perineural invasion [PNI], lymphovascular invasion [LVI], differentiation, surgical resection margin, smoking, and drinking habits) were obtained from medical records, including surgical records, biopsy reports, and radiographic images. TNM classification was performed based on the AJCC 8th Oral Cancer Classification Criteria published in 2017, and pathological TNM (pTNM) data were used in this study. The criteria for postoperative RT included T3 or T4 tumors, multiple metastatic neck nodes, or a close resection margin within 5 mm. Adjuvant CCRT was considered when a positive resection margin or extra-nodular extension (ENE) was observed.
After treatment, follow-up procedures included neck enhanced computed tomography (CT), posteroanterior chest X-ray (Chest PA), and chest CT at intervals of three to six months, and positron emission tomography (PET)-CT at one-year intervals. Any case confirmed by imaging or biopsy during follow-up was considered recurrence. The patient’s death was confirmed based on medical records. Causes of death included disease progression, other primary cancers, or underlying diseases. The overall survival rate (OS) was calculated as the proportion of patients who survived from the day of surgery. Furthermore, the disease-free survival rate (DFS) was defined as the proportion of patients who survived without any signs or symptoms of recurrence after surgery.
Prism 9 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. The univariate analysis of five-year OS and DFS were performed using the Kaplan–Meier survival analysis, and the survival rates according to clinicopathologic factors were compared respectively. The statistical significance of the survival rate by risk factor was investigated using the log-rank test. For the multivariable analysis, a Cox Proportional Hazard Model analysis was used. In both analyses, statistical significance was considered when P<0.05.
A total of 407 patients received surgical treatment at the NCC during the study period. The distribution of the clinical and pathological data is shown in Table 1. The patients included 261 male patients (64.1%) and 146 female patients (35.9%). The most common primary site was the tongue (199 patients, 48.9%), followed by the lower gingiva, buccal cheek, retromolar trigone (RMT), upper gingiva, floor of mouth (FOM), lip, palate, and others. A total of 146 patients (35.9%) had early-stage disease, whereas 261 (64.1%) had advanced-stage disease. The disease recurred in 138 patients (33.9%), while 269 patients (66.1%) remained recurrence free.
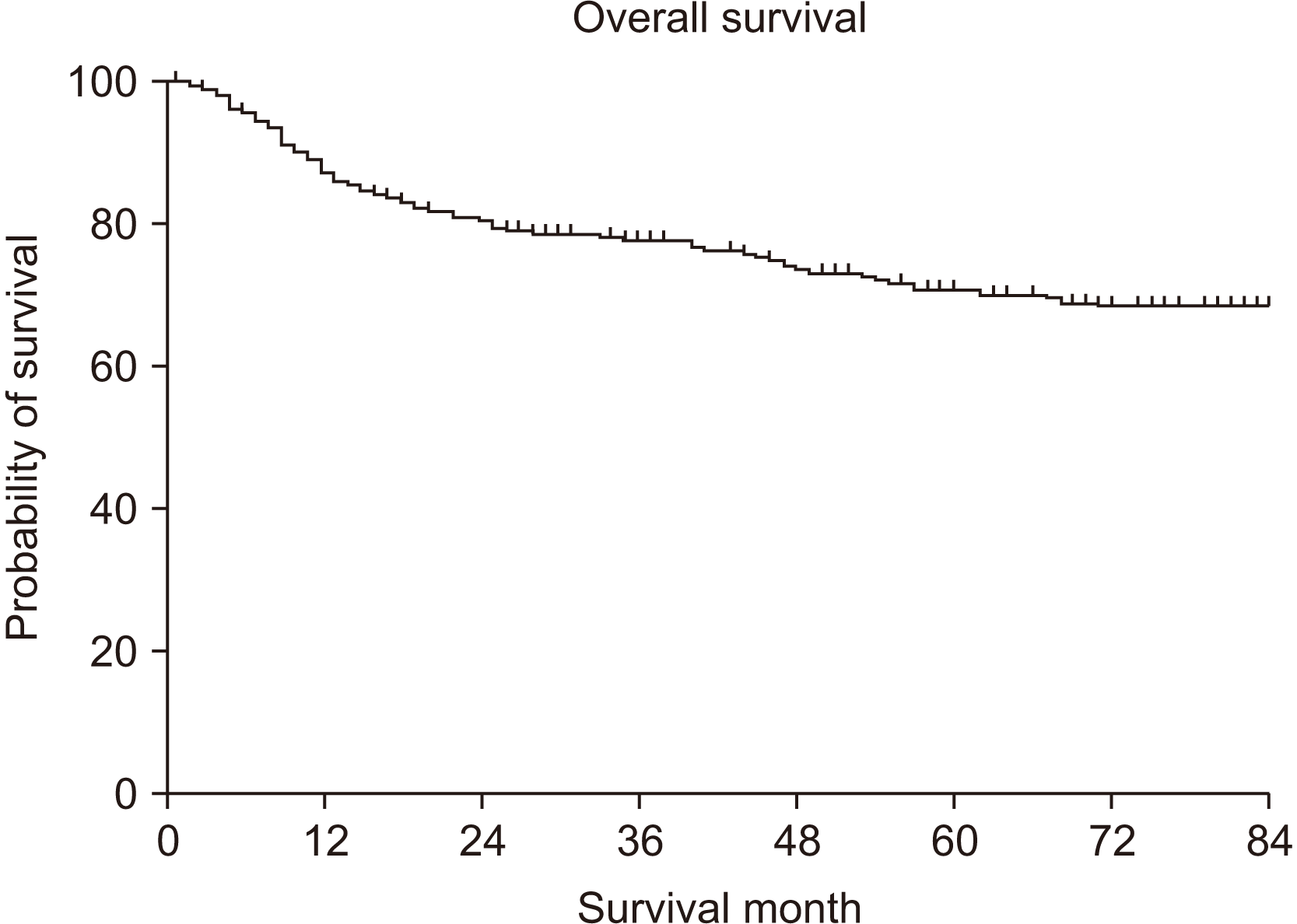
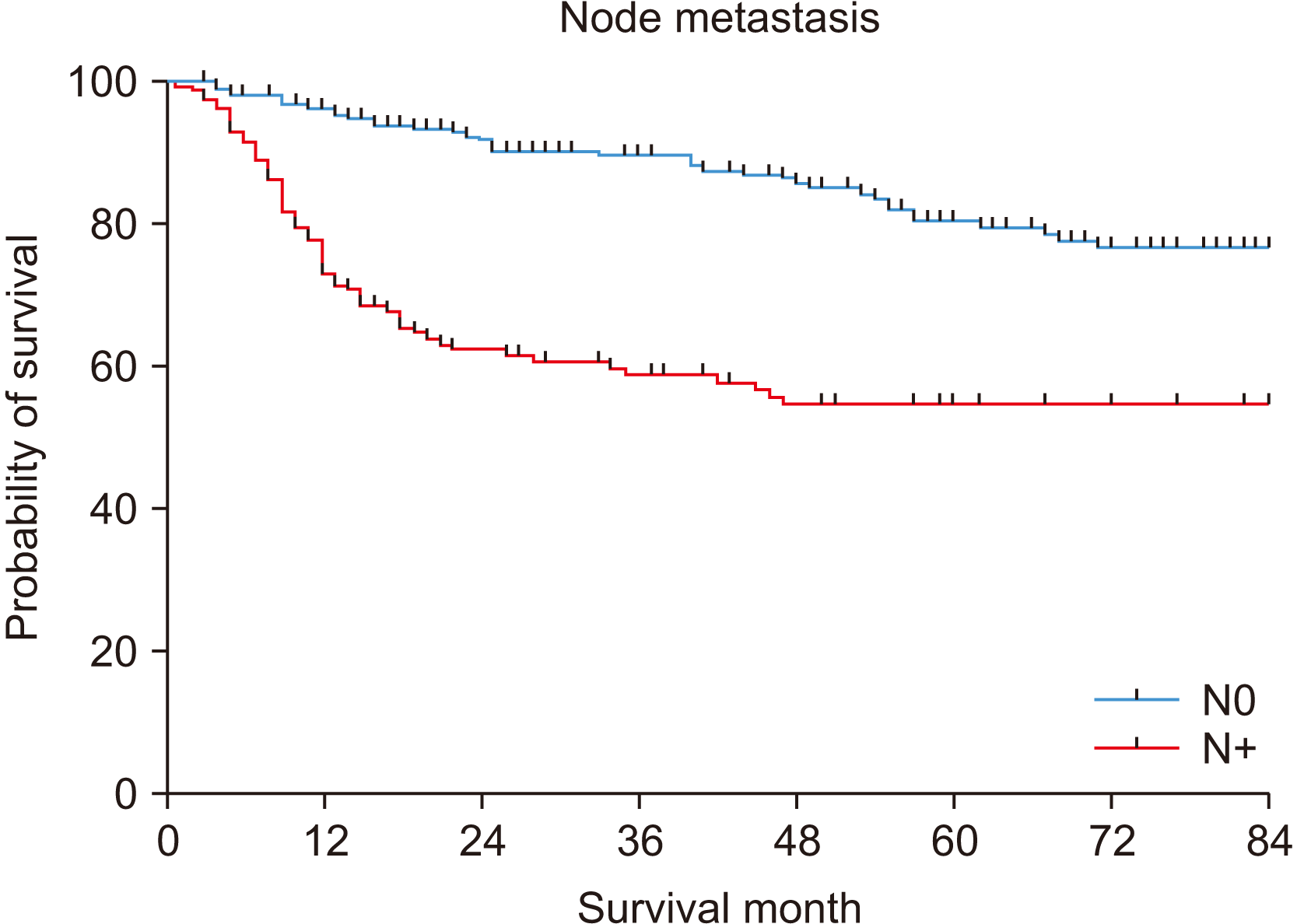
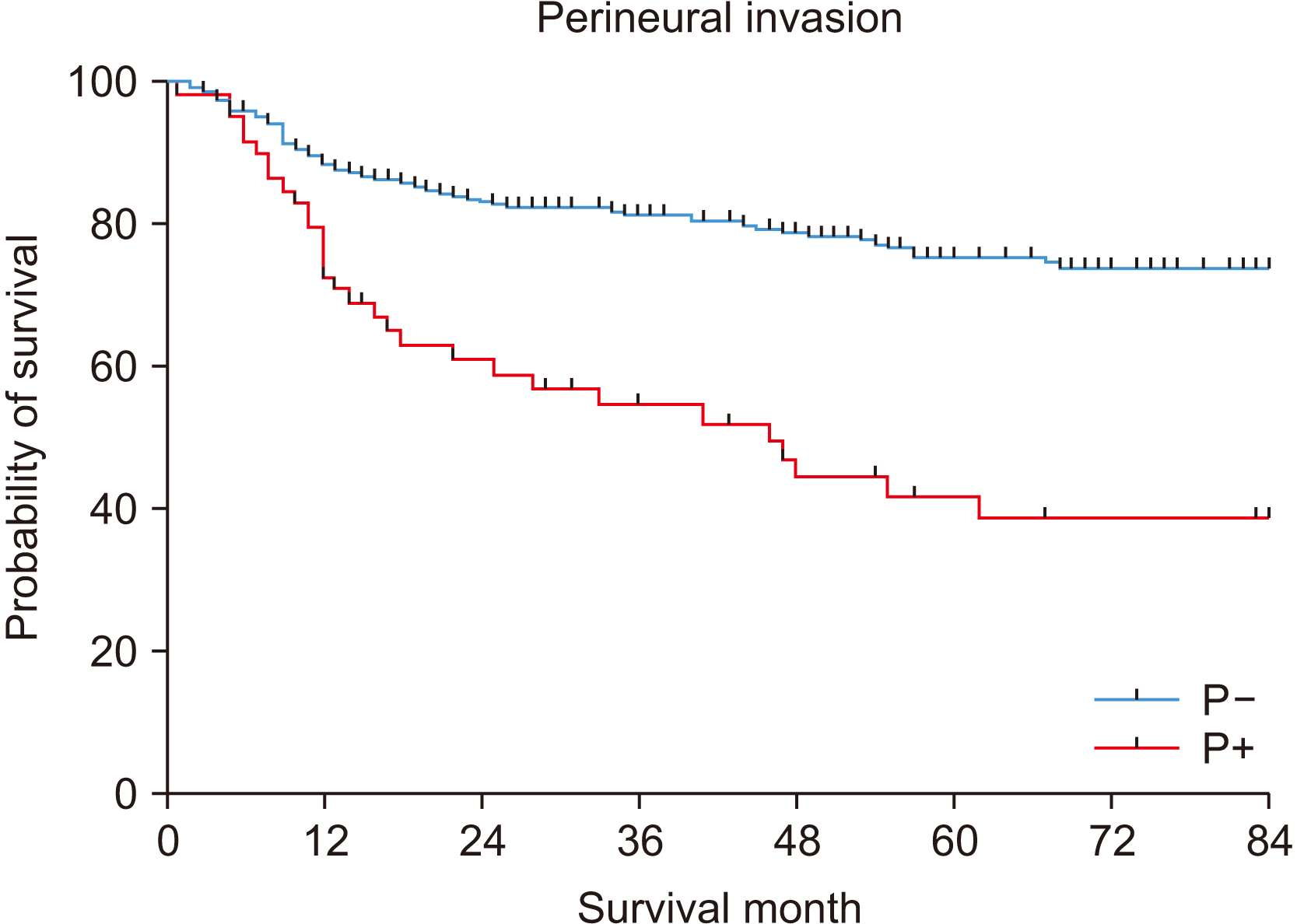
The five-year OS was 70.7%.(Fig. 1) In the univariate analysis, T stage, node metastasis, TNM stage, PNI, LVI, differentiation, surgical resection margin, and smoking were significantly associated with a poor prognosis.(Table 2) In particular, node metastasis showed differences of 80.5% and 54.7% for N0 and N+, respectively (P<0.001).(Fig. 2) PNI also showed a significant difference in survival rates of 75.6% and 41.9% for P– and P+, respectively (P<0.001).(Fig. 3)
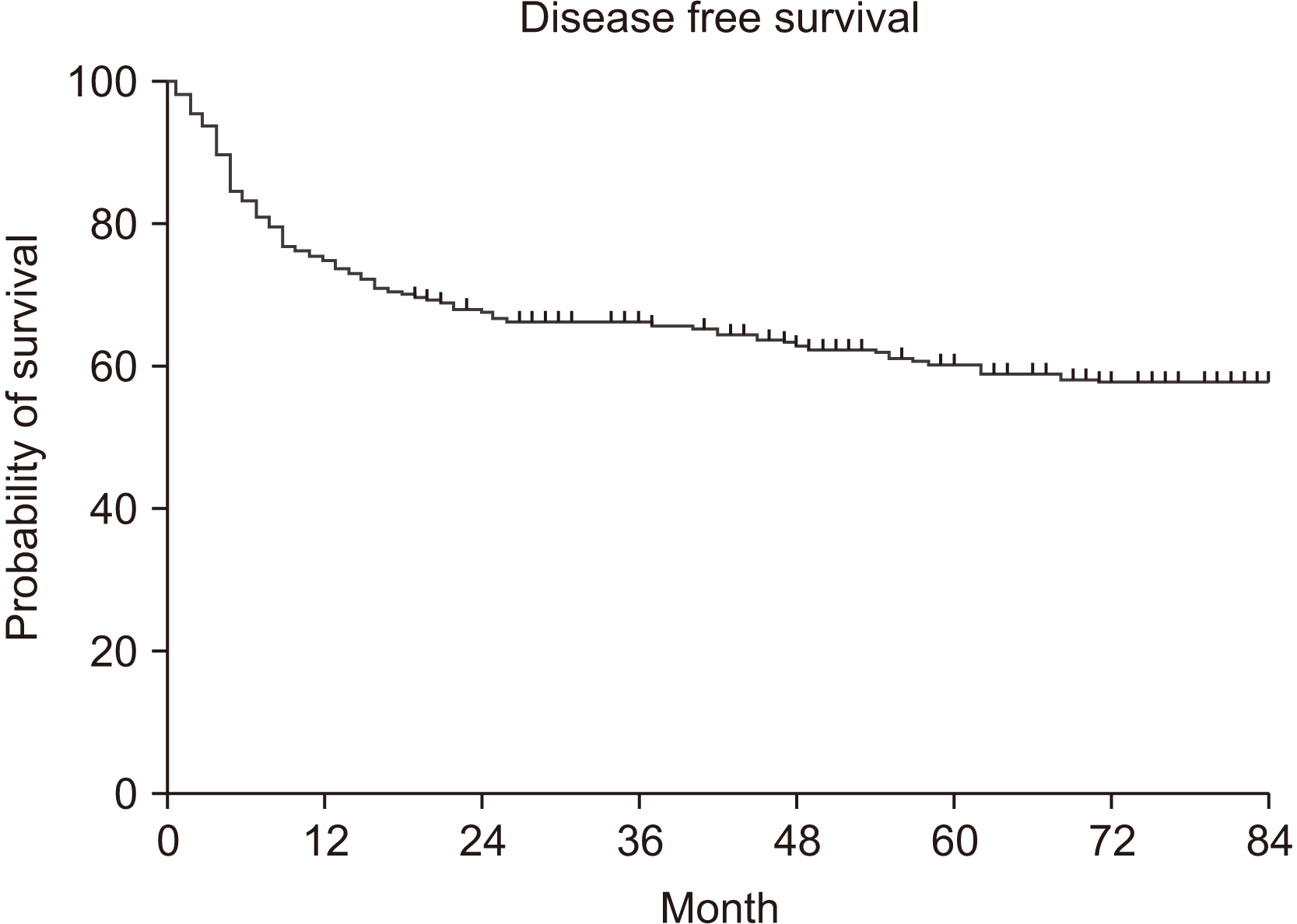
The five-year DFS was 60.6%.(Fig. 4) Factors indicating a significant difference in DFS were T stage, node metastasis, TNM stage, PNI, LVI, differentiation, and surgical resection margin.(Table 3) Interestingly, there was no statistically significant difference for smoking and DFS, but there was a significant difference in OS (P=0.307).
We performed a multivariable analysis on node metastasis, TNM stage, PNI, LVI, differentiation, surgical resection margin, and smoking, which were associated with poor prognosis in the univariate analysis of OS. Among these, node metastasis (P=0.013), PNI (P=0.007), and differentiation (P=0.004) were statistically significant.(Table 4) Moreover, the multivariable analysis revealed that PNI (P=0.022) and differentiation (P=0.025) had a significant negative effect in DFS.(Table 5)
According to the 8th AJCC classification, the factors affecting OSCC staging are tumor size, depth of invasion, number of metastatic nodes, location of the node (ipsilateral/contralateral), ENE, and distant metastasis6. In this study, the OS for each T stage was T1 (84.5%), T2 (74.9%), T3 (62.3%), and T4 (60.7%), with statistically significant differences (P<0.001). Additionally, for node metastasis, N0 (80.5%) and N+ (54.7%) showed a significant difference in OS (P<0.001). In TNM staging, there was a significant difference (P<0.001) in the univariate analysis (87.2% in the early stage and 60.8% in the advanced stage). Nonetheless, no difference was noted in the multivariable analysis (P=0.505). These data suggest that predicting the prognosis of OSCC patients based on stage alone is challenging, and other factors should be considered in the prognosis analysis.
Therefore, in this study, in addition to the TNM classification of OSCC, survival analysis was conducted based on sex, age, primary site, PNI, LVI, differentiation, surgical resection margin, smoking, and drinking to identify prognostic factors.
In our study, there were no significant differences in OS and DFS according to sex. Funk et al.8 and Leite and Koifman9 reported a higher OS for women with oral cancer, and Oh et al.10 reported that the OS of men and women was 61.51% and 81.86%, respectively. The OS of women was higher by approximately 20%, which was statistically significant8-10. However, in a study investigating patients treated surgically for OSCC, OS was 68.9% in men and 54.5% in women, although the difference was not statistically significant11. Arduino et al.12 and Mosleh-Shirazi et al.13 reported no difference in OS according to sex. In this study, young age (<40 years) showed lower OS and DFS than older age (≥40 years), although the difference was not statistically significant. Research on similar age groups demonstrated that the survival rate (61%-72.7%) in older patients was higher than that in young patients (55%-66.6%)14-16. In contrast with our data, Pytynia et al.17, Ho et al.18, and Udeabor et al.19 reported higher survival rates in young patients. Further research is required considering the inconsistent data on survival rates according to sex and age.
The oral cavity is composed of various sublocations, such as the tongue, FOM, cheek mucosa, alveolar gingiva, and lip, which have different functions and histological structures20. There is a large difference in the incidence of cancer according to its sublocation in the oral cavity. In the United States, oral cancer incidence occurs in the following order: tongue (31.9%), FOM (28.4%), retromolar area (9.3%), palate (7.7%), cheek mucosa (6.7%), lower gum (6.1%), and upper gum (2.8%)8. In this study, OSCC incidence occurred in the following order: tongue, lower gingiva, buccal cheek, RMT, upper gingiva, FOM, lip, and palate. Additionally, there was no significant difference in OS and DFS between the two groups. Similar results were found in previous studies, in which the sublocation of OSCC did not appear to affect the survival of OSCC patients8,21.
PNI is closely related to recurrence of OSCC, locoregional and distant metastasis, and overall survival22-25. In this study, PNI was also found to significantly affect survival in both the univariate and multivariable analyses. In the presence of PNI, the five-year OS rate was 41.9%, which was lower than that in the absence of PNI (75.6%). Conversely, some studies showed that the presence or absence of PNI had no significant effect on survival in stage I-II early disease or N0 disease26,27. Therefore, PNI can be considered a factor that significantly affects survival in advanced stage OSCC.
The role of LVI as a prognostic marker remains controversial28. LVI is observed in 4.9%-36.9% of OSCC cases, although conflicting results exist as to whether it significantly affects survival29-32. In this study, LVI was found in 21.7% of patients, and the results were consistent with previous studies. The univariate analysis of the overall OS and DFS showed statistical significance. However, it was not significant in the multivariable analysis; therefore, LVI was not considered a factor directly affecting survival.
In terms of tumor grade, a well-differentiated tumor (grade 1) has improved prognosis compared to moderately or poorly differentiated tumors (grade 2-3)33,34. In addition, Wang et al.35 reported that tumor grade was significantly associated with recurrence. However, previous research showed no statistical significance between tumor differentiation and survival rate36. In our study, as tumor grade increased, OS and DFS decreased, which was significant in both the univariate and multivariable analyses. These divergent results may be due to inter-observer and intra-observer variation, which may limit generalizability and reproducibility37. Nevertheless, patients with grade 3 tumors in this study showed a very low survival rate. Therefore, close observation and adjuvant therapy should be considered whenever biopsy results confirm grade 3.
The surgical resection margin was the only factor determined by the surgeon. In oral cancer, the distance from the resection margin to the tumor cells is divided into clear margin and close margin based on 5 mm, which has been accepted as a universal standard by most clinicians treating oral cancer38. In particular, the close margin or involved margin confirmed after surgery has been considered one of the factors contributing the implementation of adjuvant therapy39. However, there is controversy as to whether a 5 mm close margin predicts a poor prognosis40,41. In this study, there were significant differences in the univariate analysis for OS and DFS, but not in the multivariable analysis. This finding is consistent with other studies suggesting that the close margin threshold should be reconsidered. The quality of life of patients can be improved if the extent of resection is reduced or additional adjuvant therapy is not required. Therefore, further research and consideration is required on the criteria for close margins.
Alcohol and tobacco use are known risk factors for oral cancer42. In the oral cavity, there was no significant difference in the case of a small amount of alcohol consumption. However, the relative risk increased as the amount of alcohol consumption increased43. Nonetheless, no correlation was noted between alcohol consumption and prognosis44. In this study, there was little difference in OS and DFS between alcohol consumers and non-consumers. Therefore, alcohol itself is not a prognostic factor. Smoking had a significant effect on lowering the survival rate in the univariate analysis; however, the correlation was low in the multivariable analysis. Other studies also reported no significant effect between smoking and survival11,21,45. Although carcinogens increase the risk of cancer, they do not affect prognosis after treatment.
Our study has several limitations. A potential bias may have arisen in the retrospective study design. Our clinic follows standardized guidelines, although these may vary based on the experience of the surgeons. The histopathological characteristics of the analyzed specimens have been documented over a long period by several pathologists. Therefore, standardization of pathological evaluation should be considered in future research.
In this study, we retrospectively analyzed prognostic factors of OSCC patients after surgery. In multivariable analysis, PNI and differentiation were associated with poor OS and DFS. Node metastasis showed a statistically significant difference only in DFS. According to univariate analysis, LVI, surgical resection margin and smoking habit affected poor prognosis. Therefore, if these findings are observed pre or postoperatively, it is necessary to consider close observation and adjuvant therapy to increase the survival rate.
Notes
Authors’ Contributions
Y.S.C. participated in design of the study and statistical analysis, and wrote the manuscript. M.G.K. participated in data collection. J.H.L., J.Y.P., and S.W.C. participated in the study design, coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. 2021; Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–49. https://doi.org/10.3322/caac.21660. DOI: 10.3322/caac.21660. PMID: 33538338.

2. Rivera C. 2015; Essentials of oral cancer. Int J Clin Exp Pathol. 8:11884–94. https://doi.org/10.5281/zenodo.192487. PMID: 26617944. PMCID: PMC4637760.

3. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. 2022; ; Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. 54:330–44. https://doi.org/10.4143/crt.2022.128. DOI: 10.4143/crt.2022.128. PMID: 35313102. PMCID: PMC9016309.

4. Sklenicka S, Gardiner S, Dierks EJ, Potter BE, Bell RB. 2010; Survival analysis and risk factors for recurrence in oral squamous cell carcinoma: does surgical salvage affect outcome? J Oral Maxillofac Surg. 68:1270–5. https://doi.org/10.1016/j.joms.2009.11.016. DOI: 10.1016/j.joms.2009.11.016. PMID: 20347201.

5. Amit M, Yen TC, Liao CT, Chaturvedi P, Agarwal JP, Kowalski LP, et al. 2013; International Consortium for Outcome Research (ICOR) in Head and Neck Cancer. Improvement in survival of patients with oral cavity squamous cell carcinoma: an international collaborative study. Cancer. 119:4242–8. https://doi.org/10.1002/cncr.28357. DOI: 10.1002/cncr.28357. PMID: 24114787.

6. Almangush A, Mäkitie AA, Triantafyllou A, de Bree R, Strojan P, Rinaldo A, et al. 2020; Staging and grading of oral squamous cell carcinoma: an update. Oral Oncol. 107:104799. https://doi.org/10.1016/j.oraloncology.2020.104799. DOI: 10.1016/j.oraloncology.2020.104799. PMID: 32446214.

7. Patel SG, Lydiatt WM. 2008; Staging of head and neck cancers: is it time to change the balance between the ideal and the practical? J Surg Oncol. 97:653–7. https://doi.org/10.1002/jso.21021. DOI: 10.1002/jso.21021. PMID: 18493945.

8. Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT. 2002; Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 24:165–80. https://doi.org/10.1002/hed.10004. DOI: 10.1002/hed.10004. PMID: 11891947.

9. Leite IC, Koifman S. 1998; Survival analysis in a sample of oral cancer patients at a reference hospital in Rio de Janeiro, Brazil. Oral Oncol. 34:347–52. https://doi.org/10.1016/s1368-8375(98)00019-0. DOI: 10.1016/S1368-8375(98)00019-0. PMID: 9861339.

10. Oh MS, Kang SH, Kim HJ, Zhenglin Z, Ryu JI, Nam W, et al. 2009; Overall five-year survival rate in squamous cell carcinoma of oral cavity. J Korean Assoc Oral Maxillofac Surg. 35:83–8.
11. Sim YC, Hwang JH, Ahn KM. 2019; Overall and disease-specific survival outcomes following primary surgery for oral squamous cell carcinoma: analysis of consecutive 67 patients. J Korean Assoc Oral Maxillofac Surg. 45:83–90. https://doi.org/10.5125/jkaoms.2019.45.2.83. DOI: 10.5125/jkaoms.2019.45.2.83. PMID: 31106136. PMCID: PMC6502750.

12. Arduino PG, Carrozzo M, Chiecchio A, Broccoletti R, Tirone F, Borra E, et al. 2008; Clinical and histopathologic independent prognostic factors in oral squamous cell carcinoma: a retrospective study of 334 cases. J Oral Maxillofac Surg. 66:1570–9. https://doi.org/10.1016/j.joms.2007.12.024. DOI: 10.1016/j.joms.2007.12.024. PMID: 18634942.

13. Mosleh-Shirazi MS, Mohammadianpanah M, Mosleh-Shirazi MA. 2009; Squamous cell carcinoma of the oral tongue: a 25-year, single institution experience. J Laryngol Otol. 123:114–20. https://doi.org/10.1017/S0022215108003186. DOI: 10.1017/S0022215108003186. PMID: 18588737.

14. Fang QG, Shi S, Liu FY, Sun CF. 2014; Tongue squamous cell carcinoma as a possible distinct entity in patients under 40 years old. Oncol Lett. 7:2099–102. https://doi.org/10.3892/ol.2014.2054. DOI: 10.3892/ol.2014.2054. PMID: 24932296. PMCID: PMC4049723.

15. Soudry E, Preis M, Hod R, Hamzany Y, Hadar T, Bahar G, et al. 2010; Squamous cell carcinoma of the oral tongue in patients younger than 30 years: clinicopathologic features and outcome. Clin Otolaryngol. 35:307–12. https://doi.org/10.1111/j.1749-4486.2010.02164.x. DOI: 10.1111/j.1749-4486.2010.02164.x. PMID: 20738340.

16. Park JO, Sun DI, Cho KJ, Joo YH, Yoo HJ, Kim MS. 2010; Clinical outcome of squamous cell carcinoma of the tongue in young patients: a stage-matched comparative analysis. Clin Exp Otorhinolaryngol. 3:161–5. https://doi.org/10.3342/ceo.2010.3.3.161. DOI: 10.3342/ceo.2010.3.3.161. PMID: 20978546. PMCID: PMC2958509.

17. Pytynia KB, Grant JR, Etzel CJ, Roberts D, Wei Q, Sturgis EM. 2004; Matched analysis of survival in patients with squamous cell carcinoma of the head and neck diagnosed before and after 40 years of age. Arch Otolaryngol Head Neck Surg. 130:869–73. https://doi.org/10.1001/archotol.130.7.869. DOI: 10.1001/archotol.130.7.869. PMID: 15262765.

18. Ho HC, Lee MS, Hsiao SH, Hwang JH, Hung SK, Chou P, et al. 2008; Squamous cell carcinoma of the oral cavity in young patients: a matched-pair analysis. Eur Arch Otorhinolaryngol. 265 Suppl 1:S57–61. https://doi.org/10.1007/s00405-007-0496-5. DOI: 10.1007/s00405-007-0496-5. PMID: 17957378.

19. Udeabor SE, Rana M, Wegener G, Gellrich NC, Eckardt AM. 2012; Squamous cell carcinoma of the oral cavity and the oropharynx in patients less than 40 years of age: a 20-year analysis. Head Neck Oncol. 4:28. https://doi.org/10.1186/1758-3284-4-28. DOI: 10.1186/1758-3284-4-28. PMID: 22647235. PMCID: PMC3414801.

20. Cruchley AT, Bergmeier LA. Bergmeier LA, editor. 2018. Structure and functions of the oral mucosa. Oral mucosa in health and disease: a concise handbook. Springer;Cham: p. 1–18. DOI: 10.1007/978-3-319-56065-6_1.

21. Choi KK, Kim MJ, Yun PY, Lee JH, Moon HS, Lee TR, et al. 2006; Independent prognostic factors of 861 cases of oral squamous cell carcinoma in Korean adults. Oral Oncol. 42:208–17. https://doi.org/10.1016/j.oraloncology.2005.07.005. DOI: 10.1016/j.oraloncology.2005.07.005. PMID: 16249114.

22. Tadbir AA, Ashraf MJ, Sardari Y. 2009; Prognostic significance of stromal eosinophilic infiltration in oral squamous cell carcinoma. J Craniofac Surg. 20:287–9. https://doi.org/10.1097/SCS.0b013e318199219b. DOI: 10.1097/SCS.0b013e318199219b. PMID: 19218858.

23. Woolgar JA. 2006; Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 42:229–39. https://doi.org/10.1016/j.oraloncology.2005.05.008. DOI: 10.1016/j.oraloncology.2005.05.008. PMID: 16150633.

24. Rahima B, Shingaki S, Nagata M, Saito C. 2004; Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 97:423–31. https://doi.org/10.1016/j.tripleo.2003.10.014. DOI: 10.1016/j.tripleo.2003.10.014. PMID: 15088027.

25. Soo KC, Carter RL, O'Brien CJ, Barr L, Bliss JM, Shaw HJ. 1986; Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 96:1145–8. https://doi.org/10.1288/00005537-198610000-00015. DOI: 10.1288/00005537-198610000-00015. PMID: 3762289.

26. Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, Lee LY, et al. 2008; Does adjuvant radiation therapy improve outcomes in pT1-3N0 oral cavity cancer with tumor-free margins and perineural invasion? Int J Radiat Oncol Biol Phys. 71:371–6. https://doi.org/10.1016/j.ijrobp.2007.10.015. DOI: 10.1016/j.ijrobp.2007.10.015. PMID: 18474310.

27. Chen TC, Wang CP, Ko JY, Yang TL, Hsu CW, Yeh KA, et al. 2013; The impact of perineural invasion and/or lymphovascular invasion on the survival of early-stage oral squamous cell carcinoma patients. Ann Surg Oncol. 20:2388–95. https://doi.org/10.1245/s10434-013-2870-4. DOI: 10.1245/s10434-013-2870-4. PMID: 23361897.

28. Mascitti M, Togni L, Caponio VCA, Zhurakivska K, Bizzoca ME, Contaldo M, et al. 2022; Lymphovascular invasion as a prognostic tool for oral squamous cell carcinoma: a comprehensive review. Int J Oral Maxillofac Surg. 51:1–9. https://doi.org/10.1016/j.ijom.2021.03.007. DOI: 10.1016/j.ijom.2021.03.007. PMID: 33814227.

29. Ding D, Stokes W, Eguchi M, Hararah M, Sumner W, Amini A, et al. 2019; Association between lymph node ratio and recurrence and survival outcomes in patients with oral cavity cancer. JAMA Otolaryngol Head Neck Surg. 145:53–61. https://doi.org/10.1001/jamaoto.2018.2974. DOI: 10.1001/jamaoto.2018.2974. PMID: 30452499. PMCID: PMC6439806.

30. Chang WC, Chang CF, Li YH, Yang CY, Su RY, Lin CK, et al. 2019; A histopathological evaluation and potential prognostic implications of oral squamous cell carcinoma with adverse features. Oral Oncol. 95:65–73. https://doi.org/10.1016/j.oraloncology.2019.06.012. DOI: 10.1016/j.oraloncology.2019.06.012. PMID: 31345396.

31. Adel M, Kao HK, Hsu CL, Huang JJ, Lee LY, Huang Y, et al. 2015; Evaluation of lymphatic and vascular invasion in relation to clinicopathological factors and treatment outcome in oral cavity squamous cell carcinoma. Medicine (Baltimore). 94:e1510. https://doi.org/10.1097/MD.0000000000001510. DOI: 10.1097/MD.0000000000001510. PMID: 26512553. PMCID: PMC4985367.

32. Kim HC, Kusukawa J, Kameyama T. 1993; Clinicopathologic parameters in predicting cervical nodal metastasis in early squamous cell carcinoma of the oral cavity. Kurume Med J. 40:183–92. https://doi.org/10.2739/kurumemedj.40.183. DOI: 10.2739/kurumemedj.40.183. PMID: 8007626.

33. Kademani D, Bell RB, Bagheri S, Holmgren E, Dierks E, Potter B, et al. 2005; Prognostic factors in intraoral squamous cell carcinoma: the influence of histologic grade. J Oral Maxillofac Surg. 63:1599–605. https://doi.org/10.1016/j.joms.2005.07.011. DOI: 10.1016/j.joms.2005.07.011. PMID: 16243176.

34. Lin NC, Hsu JT, Tsai KY. 2020; Survival and clinicopathological characteristics of different histological grades of oral cavity squamous cell carcinoma: a single-center retrospective study. PLoS One. 15:e0238103. https://doi.org/10.1371/journal.pone.0238103. DOI: 10.1371/journal.pone.0238103. PMID: 32841288. PMCID: PMC7447052.

35. Wang B, Zhang S, Yue K, Wang XD. 2013; The recurrence and survival of oral squamous cell carcinoma: a report of 275 cases. Chin J Cancer. 32:614–8. https://doi.org/10.5732/cjc.012.10219. DOI: 10.5732/cjc.012.10219. PMID: 23601241. PMCID: PMC3845544.

36. Kim KY, Zhang X, Kim SM, Lee BD, Cha IH. 2017; A combined prognostic factor for improved risk stratification of patients with oral cancer. Oral Dis. 23:91–6. https://doi.org/10.1111/odi.12579. DOI: 10.1111/odi.12579. PMID: 27588367.

37. Sawair FA, Irwin CR, Gordon DJ, Leonard AG, Stephenson M, Napier SS. 2003; Invasive front grading: reliability and usefulness in the management of oral squamous cell carcinoma. J Oral Pathol Med. 32:1–9. https://doi.org/10.1034/j.1600-0714.2003.00060.x. DOI: 10.1034/j.1600-0714.2003.00060.x. PMID: 12558952.

38. Alicandri-Ciufelli M, Bonali M, Piccinini A, Marra L, Ghidini A, Cunsolo EM, et al. 2013; Surgical margins in head and neck squamous cell carcinoma: what is 'close'? Eur Arch Otorhinolaryngol. 270:2603–9. https://doi.org/10.1007/s00405-012-2317-8. DOI: 10.1007/s00405-012-2317-8. PMID: 23271033.

39. Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. 2005; Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 27:843–50. https://doi.org/10.1002/hed.20279. DOI: 10.1002/hed.20279. PMID: 16161069.

40. Zanoni DK, Migliacci JC, Xu B, Katabi N, Montero PH, Ganly I, et al. 2017; A proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol Head Neck Surg. 143:555–60. https://doi.org/10.1001/jamaoto.2016.4238. DOI: 10.1001/jamaoto.2016.4238. PMID: 28278337. PMCID: PMC5473778.

41. Wong LS, McMahon J, Devine J, McLellan D, Thompson E, Farrow A, et al. 2012; Influence of close resection margins on local recurrence and disease-specific survival in oral and oropharyngeal carcinoma. Br J Oral Maxillofac Surg. 50:102–8. https://doi.org/10.1016/j.bjoms.2011.05.008. DOI: 10.1016/j.bjoms.2011.05.008. PMID: 21742422.

42. Chamoli A, Gosavi AS, Shirwadkar UP, Wangdale KV, Behera SK, Kurrey NK, et al. 2021; Overview of oral cavity squamous cell carcinoma: risk factors, mechanisms, and diagnostics. Oral Oncol. 121:105451. https://doi.org/10.1016/j.oraloncology.2021.105451. DOI: 10.1016/j.oraloncology.2021.105451. PMID: 34329869.

43. Boffetta P, Garfinkel L. 1990; Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology. 1:342–8. https://doi.org/10.1097/00001648-199009000-00003. DOI: 10.1097/00001648-199009000-00003. PMID: 2078609.

44. Bundgaard T, Bentzen SM, Wildt J. 1994; The prognostic effect of tobacco and alcohol consumption in intra-oral squamous cell carcinoma. Eur J Cancer B Oral Oncol. 30B:323–8. https://doi.org/10.1016/0964-1955(94)90033-7. DOI: 10.1016/0964-1955(94)90033-7. PMID: 7703801.

45. Capote-Moreno A, Brabyn P, Muñoz-Guerra MF, Sastre-Pérez J, Escorial-Hernandez V, Rodríguez-Campo FJ, et al. 2020; Oral squamous cell carcinoma: epidemiological study and risk factor assessment based on a 39-year series. Int J Oral Maxillofac Surg. 49:1525–34. https://doi.org/10.1016/j.ijom.2020.03.009. DOI: 10.1016/j.ijom.2020.03.009. PMID: 32360101.

Table 1
Distribution of oral squamous cell carcinoma among patients according to clinicopathological characteristics (n=407)
Table 2
Univariate analysis for overall survival
Table 3
Univariate analysis for disease free survival
Table 4
Multivariable analysis for overall survival
Table 5
Multivariable analysis for disease free survival




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download