This article has been
cited by other articles in ScienceCentral.
Abstract
The specific muscular structure of the tongue greatly affects margin shrinkage and tumor invasion, making the optimal surgical margin controversial. This study investigated surgical margin correlated prognosis of TSCC (tongue squamous cell carcinoma) according to margin location and its value, and the histopathologic factors which are suggestive of tumor invasion. And we would like to propose defining of the surgical margin for TSCC via prognosis according to location and margin values. We reviewed 45 patients diagnosed with TSCC who visited Seoul National University Dental Hospital (SNUDH) (Seoul, Republic of Korea) from 2010 to 2019, who were managed by a single surgical team. Patient clinical and pathological data of patients were retrospectively reviewed, and in 36 out of 45 patients, the pathologic parameters including the worst pattern of invasion (WPOI) and tumor budding were investigated via diagnostic histopathology slide reading. When standardized with as 0.25 cm anterior margins, as 0.35 cm deep margin, there was no significant difference in disease specific survival (DSS) or loco-regional recurrence-free survival (LRFS). Additionally, there was a non-significant difference in DSS and LRFS at the nearest margin of 0.35 cm (PDSS=0.276, PLRFS=0.162). Aggressive WPOI and high tumor budding showed lower survival and recurrence-free survival, and there were significant differences in close margin and involved margin frequencies. In TSCC, the value and location of the surgical margin did not have a significant relationship with prognosis, but WPOI and tumor budding suggesting the pattern of muscle invasion affected survival and recurrence-free survival. WPOI and tumor budding should be considered when setting an optimal surgical margin.
Keywords: Oral tongue squamous cell carcinoma, Surgical margin, Worst pattern of invasion, Tumor budding
I. Introduction
Oral squamous cell carcinoma (OSCC) is a malignant tumor accounting for more than 90% of oral cancers and the tongue is the most common OSCC site, which accounts for about 50% or more
1. The tongue, unlike other tissues in the oral cavity, has a characteristic structure including a high content of muscle bundles and a rich lymphatic chain
2. A high proportion of muscles in the tongue make so that it can be a routes for tumoral spread through muscle fibers, thus local recurrence could be higher with muscle invasion
3. In addition to creating a specific tumor microenvironment, it can also induce lots of shrinkage at resection which is affected by tissue composition and tumor cells cohesiveness.(
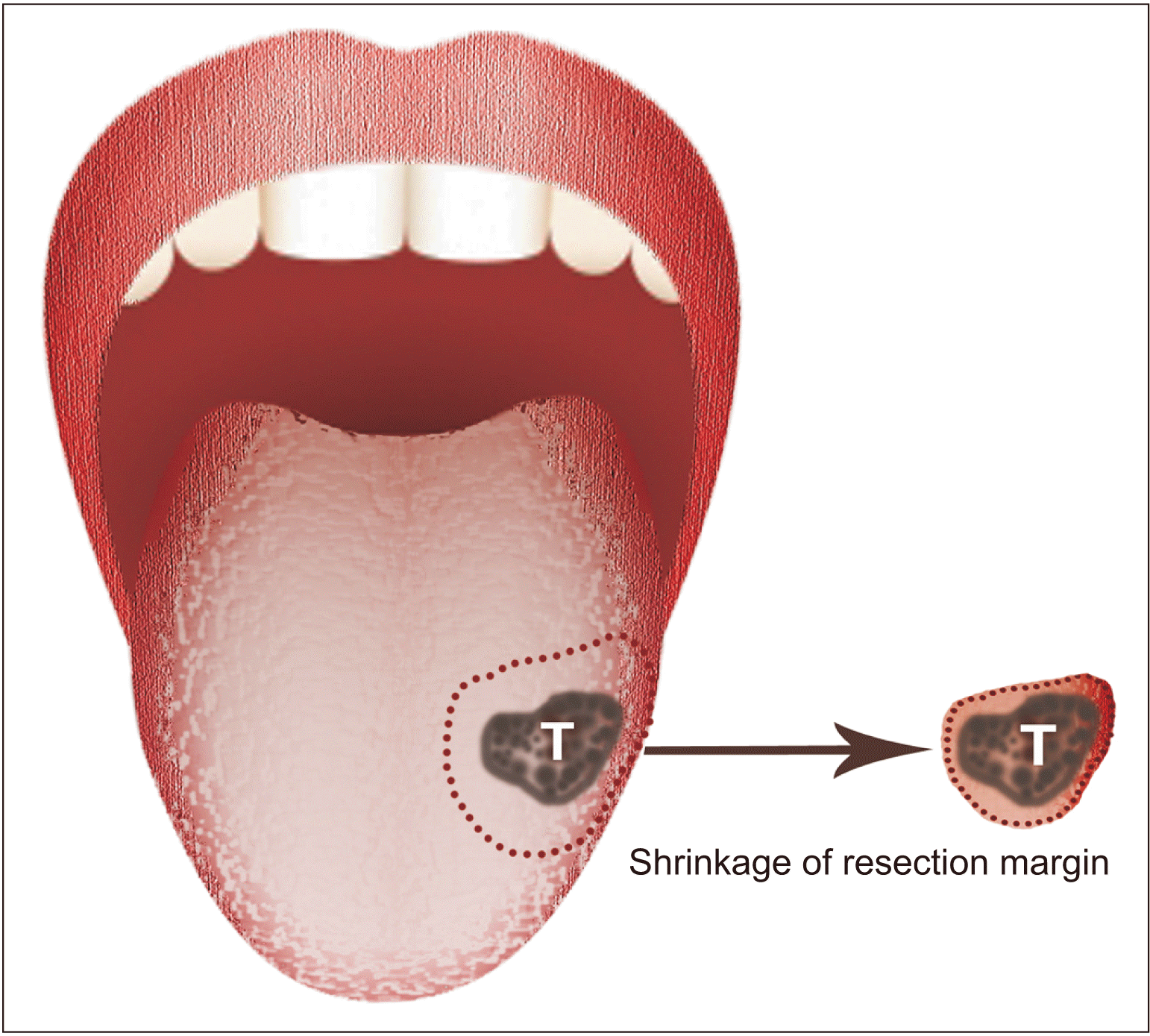
Fig. 1) As reported in previous studies
4, the tissue shrinkage in the tongue had been reported to 23.5%-42.14%, which showed greater shrinkage compared to other location of the oral cavity. The microenvironment of the medial side which is adjacent to the muscular tissue of the tongue, neurovascular bundle, and mesenchymal tissue makes the boundary of the tumor unclear, irregular. Also it causes spreading and invasion of tumor cells from the mass, inducing epithelial to mesenchymal transition and infiltration
5. These aspects make it difficult to set the surgical margin during surgery and obtain a sufficient surgical margin, and the interaction between cancer cells and the surrounding microenvironment acts as an important factor in tumor development, invasion and metastasis
5.
Fig. 1
Schematic drawing showing margin shrinkage of the tongue mass after detachment. When tumor mass was dissected from adjacent tissue, tissue shrinkage occurred. Shrinkage occurred differently according to tissue composition and anatomical site, and varied between the tongue, other oral cavities, and the medial and lateral sides of tongue.

In addition to perineural and lympho-vascular invasion and depth of invasion, current literatures suggest that histopathologic parameters like pattern of invasion (POI) and, tumor budding should be predictors of invasion, nodal metastasis and prognostic criteria
2,6-8. POI is a pathologically classified invasion pattern of the resection margin to evaluate tumor aggressiveness
6-8 and to identify the worst pattern of invasion (WPOI). Tumor budding indicates loss of cellular cohesion and, active invasive movement and was defined as a single cancer cell or a cluster of less than five cancer cells in the stroma of the invasive front. According to the guideline published by the International Tumor Budding Consensus Conference, tumor budding should be assessed using ×20 objective within the hotspot at the invasive front, and graded as low (0-4 buds), intermediate (5 buds), or high (≥5 buds)
7.
Because radical resection is the fundamental treatment for tongue squamous cell carcinoma (TSCC), and considering the characteristics of the tongue, it could be expected that setting the surgical margin with consideration of tumor invasion is directly related to good prognosis.
In this study, we assumed that the size of the recommended surgical margin would vary depending on margin location and the anatomical specificity of the tongue, and we analyzed TSCC prognosis, according to the value of each location of the surgical margin. Additionally, by estimate the correlation between histopathologic prognostic factor like WPOI, tumor budding, prognosis, and surgical margin, we suggest the surgical margin of TSCC should consider anatomical specificity, tumor environment of tongue, and histopathologic prognostic factors.
II. Materials and Methods
1. Study cohort
We reviewed 45 patients diagnosed with TSCC who visited Seoul National University Dental Hospital (SNUDH) (Seoul, Republic of Korea) from 2010 to 2019 and who were, managed by a single surgical team. This study and its access of patient records were ethically approved by the Seoul National University Institutional Review Board (S-D20170026). These patients fulfilled the following inclusion criteria: (1) complete clinical evaluation, (2) mass ablation surgery with or without radiotherapy, and exclusion criteria: (1) did not undergo surgical treatment (2) did not receive adequate follow-up.
The following clinical information of patients was retrospectively reviewed, timing of surgery, tumor stage, surgical approach, survival and local recurrence, and the adjuvant therapy after surgery. Pathologic reports were also reviewed and summarized.
2. Pathologic evaluation
The pathological features, including perineural invasion, lymphovascular invasion, and depth of invasion, which were reviewed by oral and maxillofacial pathologist, were collected.
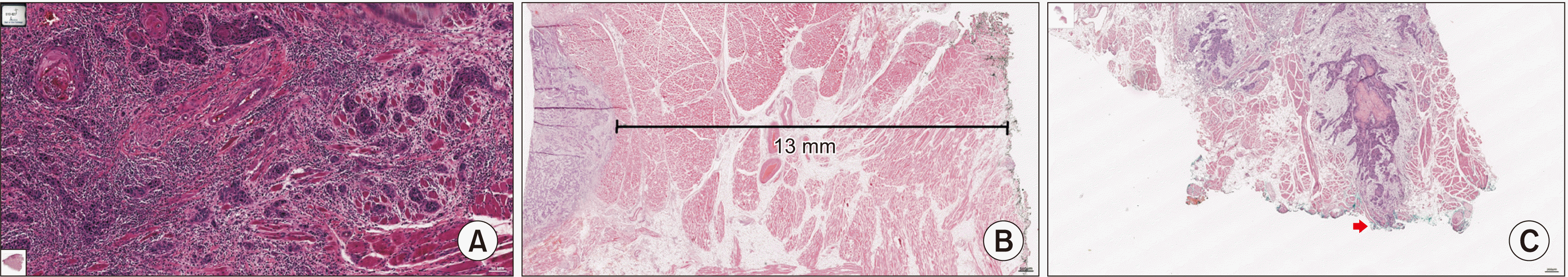
Among 45 patients, a total of 340 diagnostic histological slides from 36 patients were scanned using an Aperio CS2 (Leica Biosystems, Nussloch, Germany) and read by Case viewer software (3DHISTECH Ltd., Budapest, Hungary). POI was classified as pushing border or finger-like border or larger separate island or small separate island or tumor satellites in all H&E-stained slides, and the worst pattern was selected as a WPOI in each patient.(
Fig. 2) Tumor budding was defined as a single cell or a cluster of <5 tumor cells present in the stroma at the invasive tumor front. Slides were viewed with ×5 magnification to select the highest tumor budding area at first, and at ×20 magnification. A 0.785 mm
2 standard file size was used for the budding counts.(
Fig. 3) Images were graded as low (0-4 buds) and high (≥5 buds). Critical reviews with confirmed evaluation were carried out by an oral and maxillofacial pathologist.
Fig. 2
Pattern of invasion (POI) classified into 5 categories. Type 1 shows a broad pushing front. Type 2 shows a finger-like front (A, ×20 magnification, red arrows). Type 3 shows a larger cell group (>15-cell island, red arrow) (B, ×10 magnification). Type 4 shows smaller cell groups (≤15-cell island, red arrows), strands, or even single cells (within 1 mm from main tumor) (C, ×20 magnification). Type 5 shows satellite patterns that detached the island (red arrow) from the main tumor or island by >1 mm (D, ×10 magnification), large cell island >1 mm away from the main tumor.


Fig. 3
Histologic finding of tumor budding at invasive front area. Tumor budding was defined as a single cancer cell or a cluster of <5 cancer cells in the stroma of the invasive front (A, graded as low: 0-4 buds, intermediate or high: ≥5 buds, ×40 magnification). Posterior surgical margin of tongue mass, dyed green, showing distance from green pointed tumor margin more than 13.0 mm (B, ×40 magnification). C. Involved surgical deep margin (red arrow) of tongue, dyed with green.

3. Surgical margin evaluation
Surgical margins were evaluated based on pathology reports and diagnostic histological slides.(
Fig. 3. B, 3. C) Margin status was evaluated in 5 directions: (1) anterior; (2) posterior; (3) superior; (4) inferior; and (5) deep resection margin.
4. Statistical analysis
Surgical margin differences were evaluated by Student’s t-test, Kruskal–Wallis test, and the Mann–Whitney U test. Cut-off values of each direction of surgical margin were calculated by receiver operating characteristic (ROC) curve and Youden’s J statistic. DSS and loco-regional recurrence-free survival (LRFS) were analyzed according to the surgical margin, WPOI, and tumor budding. Univariate and multivariate regression was carried out with logistic regression and Cox-regression and hazard ratio and 95% confidence intervals (CI) were calculated for each survival and recurrence predictor. Statistical analyses were performed using IBM SPSS (ver. 26.0; IBM, Armonk, NY, USA) and P-values <0.05 were considered statistically significant.
III. Results
1. Demographics
A total of 45 patients were investigated, and according to the T-stage classification of the American Joint Committee on Cancer (AJCC) classification, T
1 (16 patients), T
2 (24 patients), T
3 (2 patients), and T
4 (3 patients) were included. Fifteen patients underwent transoral partial glossectomy only and 30 patients underwent neck dissection and glossectomy. Among patients who underwent neck dissection, 23 patients were also treated with free flap reconstruction. The mean invasion depth value was 0.886±0.61 cm and statistically significant difference according to T-stage was observed (
P=0.001). Seventeen patients were treated with adjuvant therapy after surgery; 11 patients were treated with postoperative radiotherapy (PORT); 3 patients were treated with postoperative concurrent chemo-radiotherapy (POCCRT); and 3 patients were undergoing further resection surgery. There was a statistically significant difference in postoperative adjuvant therapy strategy according to T-stage (
P=0.010). Also, the group that received adjuvant therapy showed a higher disease-specific survival rate than the group that did not, and the difference was a statistically significant (
P=0.045). There were 3 patients who received neo-adjuvant therapy, all 3 died due to disease, and 2 had recurrence.(
Table 1)
Table 1
Demographic details according to comparison with T-stage
|
Characteristic |
Overall (n=45) |
T1 (n=16) |
T2 (n=24) |
T3 (n=2) |
T4 (n=3) |
P-value |
|
Sex, male:female |
1:1.37 |
1:1.29 |
1:1.67 |
1:1.00 |
1:0.50 |
0.650 |
|
Mean age (yr) |
60.20 |
57.13 |
64.44 |
71.00 |
70.33 |
0.212 |
|
N-stage |
|
|
|
|
|
0.021*
|
|
N0
|
30 (66.7) |
13 (81.3) |
16 (66.7) |
1 (50.0) |
- |
|
|
N1
|
4 (8.9) |
1 (6.3) |
3 (12.5) |
- |
- |
|
|
N2
|
11 (24.4) |
2 (12.5) |
5 (20.8) |
1 (50.0) |
3 (100) |
|
|
Surgery |
|
|
|
|
|
|
|
Only transoral |
15 (33.3) |
9 (56.3) |
6 (25.0) |
- |
- |
|
|
With ND |
30 (66.7) |
7 (43.8) |
18 (75.0) |
2 (100) |
3 (100) |
0.032*
|
|
With reconstruction |
23 (51.1) |
6 (37.5) |
12 (50.0) |
2 (100) |
3 (100) |
0.054 |
|
Mean depth of invasion (cm) |
0.886 |
0.459 |
0.875 |
1.45 |
1.733 |
0.001*
|
|
Meta lymph node ratio |
0.10 |
0.03 |
0.21 |
0.05 |
0.24 |
|
|
ENE |
6 (13.3) |
1 (6.3) |
3 (12.5) |
0 |
2 (66.7) |
|
|
PNI |
9 (20.0) |
0 |
7 (29.2) |
1 (50.0) |
1 (33.3) |
|
|
LVI |
2 (4.4) |
0 |
0 |
0 |
2 (66.7) |
|
|
WPOI |
(n=36) |
(n=13) |
(n=18) |
(n=2) |
(n=3) |
0.017*
|
|
WPOI 2 |
2 (5.6) |
1 (7.7) |
1 (5.6) |
- |
- |
|
|
WPOI 3 |
8 (22.2) |
6 (46.2) |
2 (11.1) |
- |
- |
|
|
WPOI 4 |
15 (41.7) |
5 (38.5) |
7 (38.9) |
1 (50.0) |
2 (66.7) |
|
|
WPOI 5 |
11 (30.6) |
1 (7.7) |
8 (44.4) |
1 (50.0) |
1 (33.3) |
|
|
Tumor budding |
(n=36) |
(n=13) |
(n=18) |
(n=2) |
(n=3) |
0.027*
|
|
Low (<5 cells) |
17 (47.2) |
10 (76.9) |
5 (27.8) |
1 (50.0) |
1 (33.3) |
|
|
High (≥5 cells) |
19 (52.8) |
3 (23.1) |
13 (72.2) |
1 (50.0) |
2 (66.7) |
|
|
Additional Tx |
17 (37.8) |
2 (12.5) |
11 (45.8) |
1 (50.0) |
3 (100) |
0.010* |
|
PORT |
11 (24.4) |
2 (12.5) |
7 (29.2) |
1 (50.0) |
1 (33.3) |
|
|
POCCRT |
3 (6.7) |
- |
1 (4.2) |
- |
2 (66.7) |
|
|
Further resection |
3 (6.7) |
- |
3 (12.5) |
- |
- |
|
|
Recurrence |
19 (42.2) |
5 (31.3) |
11 (45.8) |
1 (50.0) |
2 (66.7) |
0.305 |
|
Local |
3 (6.7) |
1 (6.3) |
1 (4.2) |
1 (50.0) |
- |
|
|
Regional |
12 (26.7) |
4 (25.0) |
7 (29.2) |
- |
1 (33.3) |
|
|
Distant |
4 (8.9) |
- |
3 (12.5) |
- |
1 (33.3) |
|
|
Mean follow-up period (mo) |
58.60 |
69.93 |
59.54 |
6.00 |
25.67 |
0.021*
|
|
3-yr DSS (%) |
75.56 |
93.75 |
75.00 |
0 |
33.33 |
0.004*
|
|
3-yr LRFS (%) |
46.67 |
62.50 |
41.67 |
0 |
33.33 |
0.201 |

Recurrence occurred in 19 patients, among whom local recurrence occurred in 3 patients; regional recurrence occurred in 12 patients; and distant recurrence occurred in 4 patients. The mean follow-up period for the patient group was 58.60 months, the mean 3-year disease-free survival was 75.56%, and the mean 3-year LRFS was 46.67%. There was a statistically significant difference in DSS according to T-stage (P=0.004).
2. Surgical margin
The mean surgical margins values were 0.78±0.36 cm (anterior), 0.98±0.73 cm (posterior), 0.86±0.41 cm (superior), 0.65±0.38 cm (inferior), and 0.60±0.39 cm (medial).(
Supplementary Table 1) There was no significant difference except nearest margin according to T-stage and WPOI (
PT=0.012,
PWPOI=0.031).(
Supplementary Table 2) Also, comparing the size of the surgical margins of the survivor group and the deceased group, there was a statistically significant difference between the anterior and the medial sides of the surgical margins (
PAnt=0.013,
PDeep=0.014), and there was no significant difference surgical margin between recurrence free patients and recurrence patients.(
Table 2) The cut-off value for survival was calculated and was not statistically significant; however, the cut-off value for recurrence was significantly different for anterior surgical margins (
Supplementary Table 3), although the ROC curve showed little utility.(
Fig. 4) Based on these cut-off values, DSS and LRFS were calculated according to the value of the surgical margin. In nearest margin of 0.35 cm, the group over 0.35 cm showed higher DSS and LRFS than the group below 0.35 cm, but the difference was not significance (
PDSS=0.276,
PLRFS=0.162).(
Fig. 5,
6) Also, there was no statistically significant difference in DSS and LRFS according to the location of the individual surgical margin.
Fig. 4
Receiver operating characteristic curve of cut-off value with LRFS (loco-regional recurrence-free survival). Anterior margin showed significant value (0.25 cm, P=0.013), but also a downward trend compared with the reference line, indicating low utility.
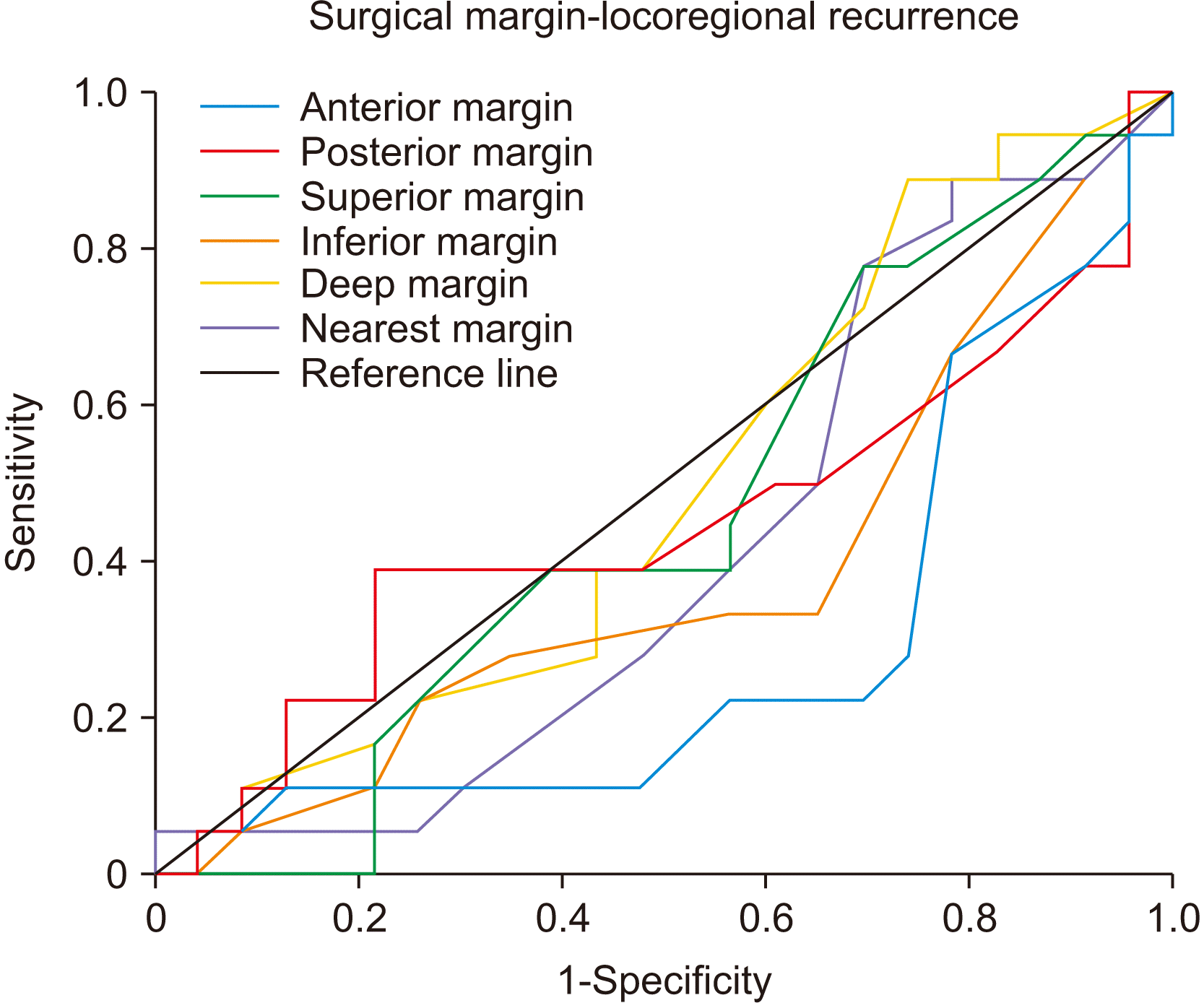

Fig. 5
Kaplan–Meier curve of DSS (disease specific survival) according to nearest margin 0.35 cm, worst pattern of invasion (WPOI), tumor budding. For disease-specific survival, the group with the nearest margin greater than 0.35 cm in the survival curve showed higher survival, but it was not statistically significant (P=0.276). And the non-aggressive WPOI group showed a 100% survival rate and showed a statistically significant difference compared with the aggressive WPOI group (P=0.03). Also, the group with low-tumor budding in the survival curve showed higher survival, but it was not statistically significant (P=0.271). Non-aggressive WPOI (WPOI types 1, 2, 3), aggressive WPOI (WPOI types 4, 5). Low tumor budding (less than 5 buds), high-tumor budding (≥5 buds). Buds defined as a single cancer cell or a cluster of less than 5 cancer cells in the stroma of the invasive front.
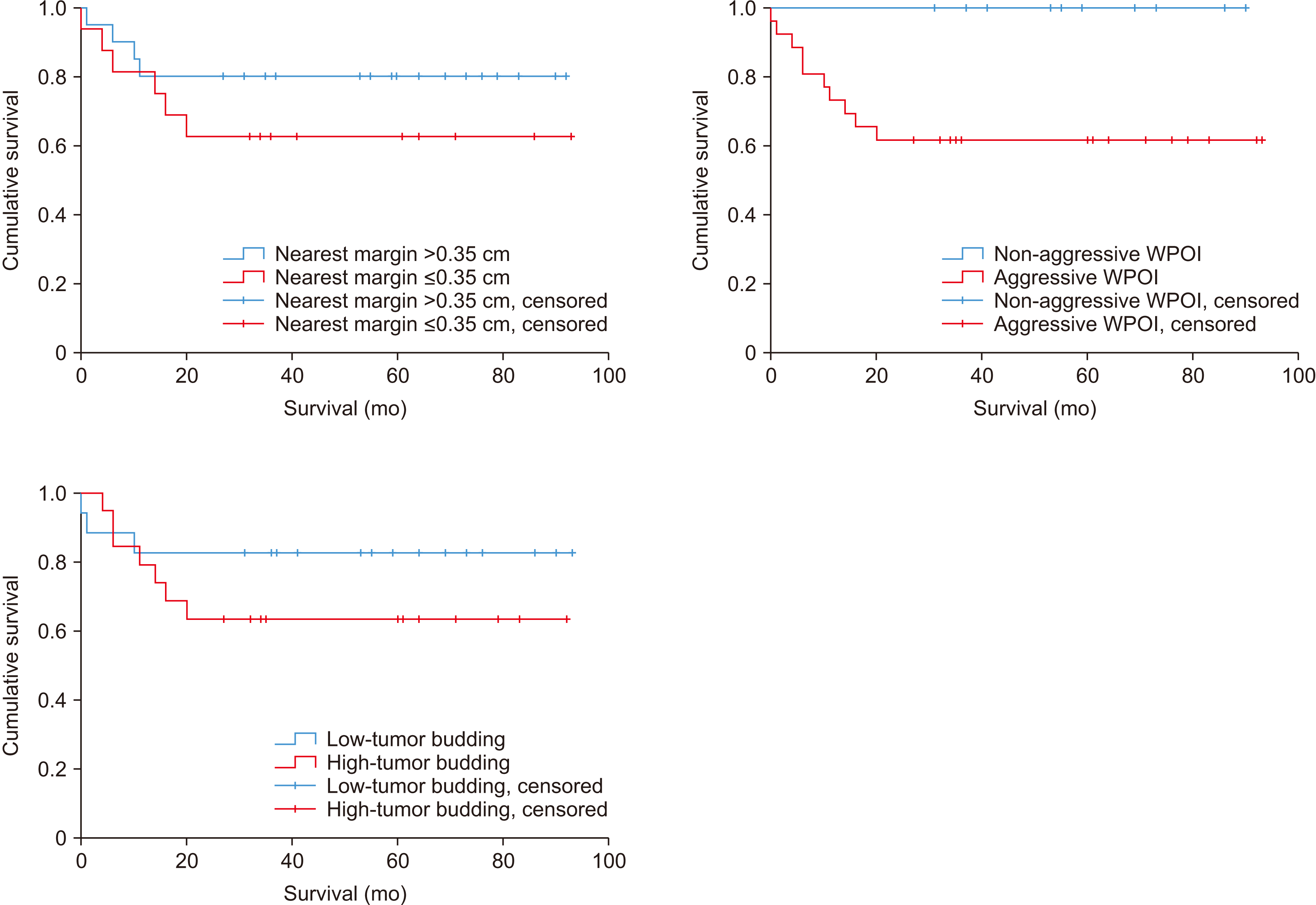

Fig. 6
Kaplan–Meier curve of loco-regional recurrence-free survival (LRFS) according to nearest margin 0.35 cm. In LRFS, the group with the margin nearest >0.35 cm in the recurrence-free survival curve showed higher recurrence-free survival, but it was not statistically significant (P=0.162), the group with non-aggressive worst pattern of invasion (WPOI) group showed higher recurrence-free survival in the curve (P=0.05), and the group with low-tumor budding had higher recurrence-free survival in the curve (P=0.024). Non-aggressive WPOI (WPOI types 1, 2, 3), aggressive WPOI (WPOI types 4, 5). Low tumor budding (less than 5 buds), high-tumor budding (≥5 buds). Buds defined as a single cancer cell or a cluster of less than 5 cancer cells in the stroma of the invasive front.
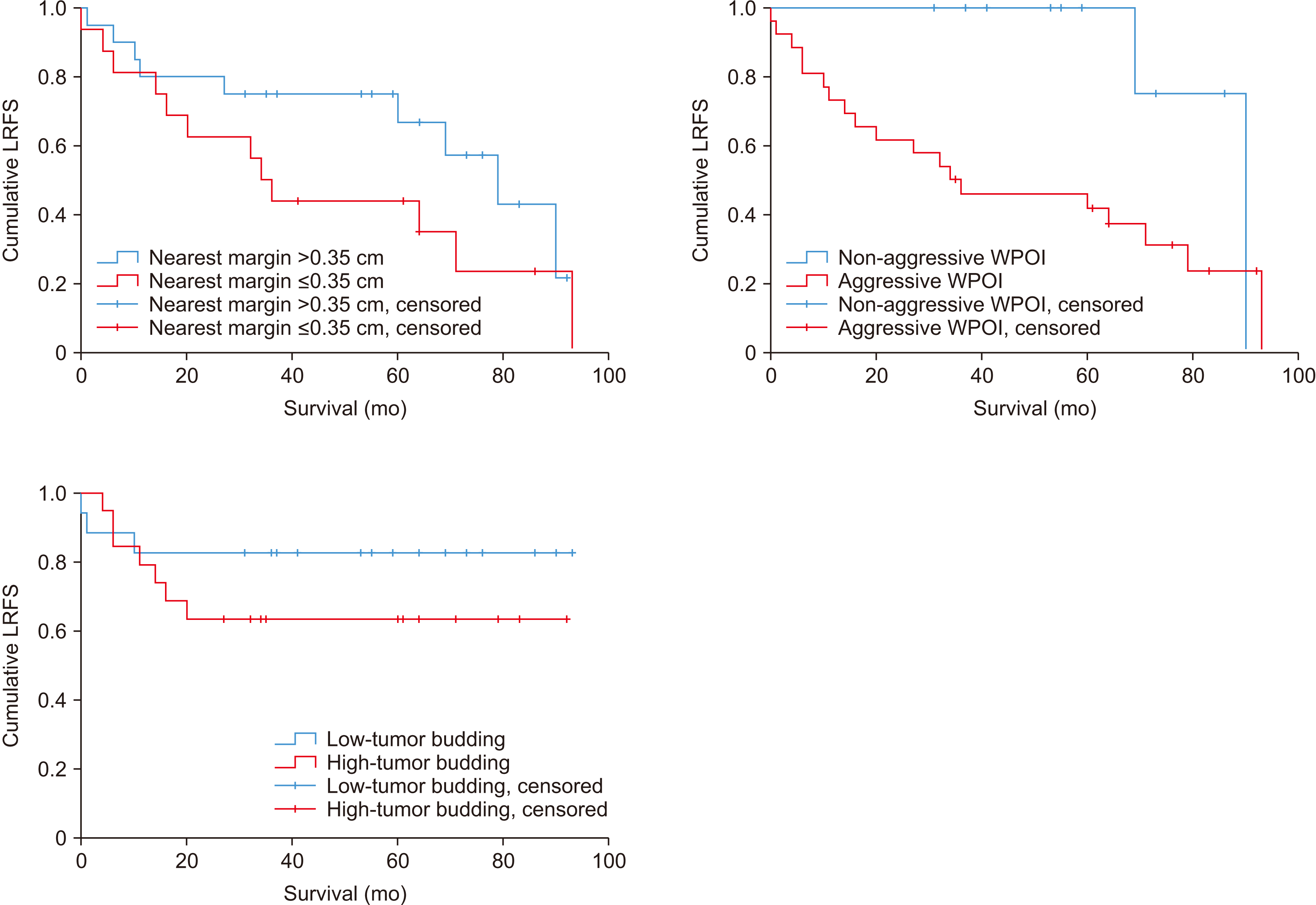

Table 2
Comparison of surgical margin, survivor and dead group, recur-free and recur group (unit: cm)
|
Surgical margin |
Survival |
|
Recurrence |
|
|
|
Alive |
Death |
P-value |
Recur-free |
Recur |
P-value |
|
Anterior |
0.83±0.26 |
0.71±0.48 |
0.013*
|
|
0.86±0.38 |
0.67±0.33 |
0.250 |
|
Posterior |
0.96±0.75 |
1.01±0.73 |
0.59 |
|
0.94±0.80 |
1.03±0.66 |
0.947 |
|
Superior |
0.84±0.37 |
0.89±0.48 |
0.67 |
|
0.90±0.49 |
0.80±0.26 |
0.057 |
|
Inferior |
0.73±0.40 |
0.52±0.31 |
0.89 |
|
0.71±0.40 |
0.57±0.34 |
0.891 |
|
Deep |
0.71±0.43 |
0.65±0.31 |
0.014*
|
|
0.67±0.44 |
0.73±0.31 |
0.176 |
|
Nearest |
0.41±0.24 |
0.37±0.24 |
0.86 |
|
0.40±0.26 |
0.40±0.21 |
0.128 |

3. Pathologic parameters
In the 36 patients, 2 patients were WPOI 2, 8 patients were WPOI 3, 15 patients were WPOI 4, and 11 patients were WPOI 5. Seventeen patients were classified into the low-tumor budding group, and 19 patients were classified into the high-tumor budding group.
In neck metastasis patients who were diagnosed during the first surgery with neck dissection or who later experienced recurrence at neck, 50% of patients (n=9) were WPOI 5, and just one patient had non-aggressive invasion (WPOI 1, 2, 3), which is a significant difference according to WPOI (
P=0.012). Also, regarding recurrence and survival there was a significant difference between non-aggressive invasion and aggressive invasion (WPOI 4, 5).(
Table 3) Among the tumor budding group, there was a significant difference in recurrence (
P=0.009), with inclusion of 70.6% patients who were in the with tumor budding group.(
Table 3)
Table 3
Incidence of neck metastasis, recurrence, survival according to WPOI and tumor budding
|
Characteristic |
WPOI |
|
Tumor budding |
|
|
|
WPOI 1, 2, 3 |
WPOI 4 |
WPOI 5 |
P-value |
w/o tumor budding |
w/ tumor budding |
P-value |
|
Neck metastasis (n=18) |
1 (5.6) |
8 (44.4) |
9 (50.0) |
0.012*
|
|
6 (33.3) |
12 (66.7) |
0.100 |
|
Recurrence (n=17) |
2 (11.8) |
7 (41.2) |
8 (47.1) |
0.023*
|
|
5 (29.4) |
12 (70.6) |
0.009* |
|
Survival (n=26) |
10 (38.5) |
9 (34.6) |
7 (26.9) |
0.016*
|
|
14 (53.8) |
12 (46.2) |
0.206 |

Regarding margin status, incidence according to WPOI and tumor budding is a shown in
Table 4. In both cases, more aggressive WPOI or higher budding is significantly associated with, worse margin status (
PWPOI=0.031,
Pbudding=0.035). DSS and LRFS according to WPOI were shown in
Figs. 5 and
6. All Kaplan–Meier graphs showed significantly poorer DSS and LRFS in aggressive WPOI (
PDSS=0.03,
PLRFS=0.05). Also, DSS and LRFS according to tumor budding, are shown in
Figs. 5 and
6. Similar to WPOI, it showed significantly poorer DSS and LRFS in high tumor budding cases, as shown, in the LRFS graph (
PDSS=0.271,
PLRFS=0.024).
Table 4
Incidence of margin status according to WPOI and tumor budding
|
Margin status |
WPOI |
|
Tumor budding |
|
|
|
WPOI 1, 2, 3 |
WPOI 4 |
WPOI 5 |
Low tumor budding |
High tumor budding |
|
Clear margin (n=8) |
3 (37.5) |
4 (50.0) |
1 (12.5) |
|
5 (62.5) |
3 (37.5) |
|
Closed margin (n=22) |
7 (31.8) |
10 (45.5) |
5 (22.7) |
|
12 (54.5) |
10 (45.5) |
|
Involved margin (n=6) |
- |
1 (16.7) |
5 (83.3) |
|
- |
6 (100) |

4. Univariate and multivariate analysis of DSS and LRFS
In disease specific survival, advanced T-stage, surgical margins <0.35 cm, high tumor budding, and aggressive WPOI had high hazard ratio, but there was only significant value for advanced T-stage (T
3, T
4), in multivariate analysis (
P=0.016).(
Table 5) Similarly, in LRFS, advanced T-stage was classified as a prognostic factor in univariate analysis (
P=0.035).(
Table 6)
Table 5
Univariate and multivariate DSS analysis using Cox-proportional hazards model
|
Variable |
Univariate |
|
Multivariate |
|
|
|
HR |
95% CI |
P-value |
HR |
95% CI |
P-value |
|
T-stage |
|
|
|
|
|
|
|
T1
|
1 (reference) |
|
|
1 (reference) |
|
|
|
T2
|
0.40 |
0.01-1.61 |
0.20 |
0.61 |
0.23-1.62 |
0.32 |
|
T3, T4
|
1.38 |
0.16-11.79 |
0.77 |
5.11 |
1.35-19.26 |
0.016*
|
|
Nearest margin |
|
|
|
|
|
|
|
≤0.35 |
1 (reference) |
|
|
1 (reference) |
|
|
|
>0.35 |
3.81 |
0.55-26.58 |
0.18 |
2.80 |
0.51-15.36 |
0.24 |
|
Tumor budding |
|
|
|
|
|
|
|
<5 |
1 (reference) |
|
|
1 (reference) |
|
|
|
≥5 |
3.86 |
0.37-40.66 |
0.26 |
1.18 |
0.43-3.26 |
0.75 |
|
WPOI |
|
|
|
|
|
|
|
WPOI 1, 2, 3 |
1 (reference) |
|
|
|
|
|
|
WPOI 4, 5 |
2.00 |
0.30-13.17 |
0.47 |
1.67 |
0.20-18.35 |
0.68 |

Table 6
Univariate and multivariate LRFS analysis using Cox-proportional hazards model
|
Variable |
Univariate |
|
Multivariate |
|
|
|
HR |
95% CI |
P-value |
HR |
95% CI |
P-value |
|
T-stage |
|
|
|
|
|
|
|
T1
|
1 (reference) |
|
|
1 (reference) |
|
|
|
T2
|
0.32 |
0.25-3.82 |
0.36 |
0.67 |
0.23-1.91 |
0.45 |
|
T3, T4
|
18.66 |
1.23-286.20 |
0.035*
|
4.90 |
1.47-16.26 |
0.09 |
|
Nearest margin |
|
|
|
|
|
|
|
≤0.35 |
1 (reference) |
|
|
1 (reference) |
|
|
|
>0.35 |
2.15 |
0.30-15.55 |
0.45 |
1.24 |
0.51-3.01 |
0.63 |
|
Tumor budding |
|
|
|
|
|
|
|
<5 |
1 (reference) |
|
|
1 (reference) |
|
|
|
≥5 |
1.65 |
0.28-9.86 |
0.58 |
2.06 |
0.41-10.47 |
0.38 |
|
WPOI |
|
|
|
|
|
|
|
WPOI 1, 2, 3 |
1 (reference) |
|
|
|
|
|
|
WPOI 4, 5 |
0.82 |
0.38-1.79 |
0.62 |
0.91 |
0.48-1.73 |
0.78 |

IV. Discussion
The optimal surgical margin of conventional OSCC has been considered to be 5 mm
9,10, but this designation is controversial
11-14. However, because tongue muscle invasion impact on tumor progression and has a relatively poor TSCC prognosis, and the optimal surgical margin of TSCC is still controversial and values have been proposed, including tongue compartment surgery
3,12-16. Zanoni et al.
12 suggested 0.22 cm as the optimal surgical margin for the TSCC cases, where the proportion of T
1 and T
2 stages reached 87%. Singh et al.
13 determined that a surgical margin of 0.76 cm was appropriate for TSCC in a patient group, where the proportion of early stage (T
1 and T
2) was about 40%. From the point of view of individual surgical margins which we expected would have different values depending on location, Lee et al.
14 reported that the posterior margin and deep margin have significant differences related to survival and recurrence. In early stage (T
1, T
2), the cut-off value of the posterior margin was 0.45 cm and, deep margin was 0.25 cm. In advanced stage (T
3, T
4), the cut-off value of the posterior margin was 0.95 cm and deep margin was 0.80 cm
14. In this study, in which T
1, T
2 stages account for 88.9% of cases, DSS, and LRFS were different at the nearest 0.35 cm margin but not significantly (
PDSS=0.276,
PLRFS=0.162). And there was no significant difference in DSS and LRFS which is standardized with an anterior margin of 0.25 cm, which showed to be a significant for the cut-off value, and a 0.35 cm deep margin, and was significantly different between the survivor group and the death group. Because of characteristic anatomical features, significantly different prognoses according to location of the surgical margin value, especially deep margin, was expected; however, there was no statistically significant difference in prognosis according to location of the surgical margin.
WPOI and tumor budding are known as parameters that reflect tumor invasiveness, and they especially correlate with loss of cellular cohesion, active invasive movement, and recurrence
6-8. In this study, WPOI and tumor budding were evaluated as pathological parameters to evaluate the effect of invasion due to the tongue’s special muscular structure in setting the surgical margin and surgery plan. According to WPOI grade, there was a significant difference in neck metastasis (
P=0.012), recurrence (
Precur=0.023) and survival (
Psurv=0.016) between non-aggressive WPOI and aggressive WPOI. Similarly, we found a significant difference in recurrence according to grade of tumor budding, it showed significant difference in recurrence (
P=0.009). Additionally, more aggressive WPOI and higher tumor budding was associated with, increased frequency of closed and involved margins 83.3% of involved margins were WPOI 5, and high tumor budding occurred in 100%. Tumor budding or satellite is often difficult to judge clinically or radiologically. Consequently, the frequency of closed or involved margins relatively increases, making it difficult to obtain sufficient surgical margins, resulting in poor prognosis. This tendency could also be also being observed in that the nearest margin decreases as WPOI increases.(
Supplementary Table 2) Considering these facts, estimation of WPOI and tumor budding before surgery is important in setting the surgical margin, and when the WPOI grade is high, a larger surgical margin is recommended and surgical planning accompanied by elective neck dissection is necessary. Because it is difficult to identify WPOI before surgery, it is necessary to infer WPOI through biopsy pattern of invasion (BPOI), and for this, biopsy results from various sites could be helpful
17.
The patient group who received adjuvant therapy showed a higher DSS than the patient group who did not receive adjuvant therapy (P=0.045), because, in most cases of closed margin patients or involved margin patients, the PORT or POCCRT procedures were performed according to AJCC guidelines. It cannot be excluded that the relationship to value, location of surgical margin and TSCC prognosis may have been influenced by this. Further studies on these aspects, with additional patient groups, are needed.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2022R1F1A1069624).
References
8. Almangush A, Bello IO, Coletta RD, Mäkitie AA, Mäkinen LK, Kauppila JH, et al. 2015; For early-stage oral tongue cancer, depth of invasion and worst pattern of invasion are the strongest pathological predictors for locoregional recurrence and mortality. Virchows Arch. 467:39–46.
https://doi.org/10.1007/s00428-015-1758-z. DOI:
10.1007/s00428-015-1758-z. PMID:
25838076.











 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download