This article has been
cited by other articles in ScienceCentral.
Abstract
Peri-implantitis is an inflammatory lesion of the periodontium surrounding an endosseous implant, with progressive loss of the supporting peri-implant bone. The main purposes of treatment for peri-implantitis due to biological factors include addressing the inflammation and restoring a healthy but reduced periodontium around the implant, similar to the treatment of periodontitis in natural teeth. The proposed treatment protocol includes surgical treatment, mainly resective surgery, after non-surgical treatment such as oral hygiene instructions, mechanical cleansing of the fixture, and general or topical antiseptic or antibiotic application according to the extent of inflammation. In this article, we present a 6-year follow-up case showing unusual marginal bone regeneration after resective surgery and decontamination of an implant surface for the treatment of peri-implantitis and discuss the possible reasons.
Go to :

초록
임플란트주위염은 임플란트의 주변 치조골의 상실을 동반한 치주조직의 염증성 병소이다. 임플란트주위염의 치료 목표는 자연치에서 발생되는 치주염의 치료와 유사하게, 염증을 해결하여 임플란트 주위 치주조직의 건강을 회복시키는 것이다. 치료 방법으로는 염증의 진행 정도에 따라 구강위생교육과 국소적 또는 전신적 방부제(antiseptics)와 항생제 처치를 동반한 지대주(fixture)의 mechanical cleansing, 삭제형 또는 재생형 골수술 등이 제안되고 있다. 본 연구에서, 임플란트 주위염의 표면처리(decontamination)와 삭제형골수술 이후, 특이한 골재생 증례의 6년 추적관찰 결과를 보고하고자 한다.
Go to :

Keywords: dental implants, peri-implantitis, bone regeneration, periosteum
색인어: 치과임플란트, 임플란트주위염, 골재생, 골막
Introduction
Dental implant treatment is a popular and mandatory treatment option for replacing missing teeth. Despite the high long-term success rate of implant prostheses, approximately 45% of patients who have undergone implant treatment suffer from peri-implantitis,
1,2 and in some cases, eventually, implant removal needs to be employed.
Peri-implantitis is an inflammatory lesion of the periodontium surrounding an endosseous implant, with progressive loss of the supporting peri-implant bone.
3 The causes of peri-implantitis include mechanical or biological factors. Mechanical factors include incorrect position and occlusal overload or interference, and biological factors include microbial infection, compression necrosis, and surgical trauma, such as overheating and overpreparation.
4
The main purposes of treatment for peri-implantitis due to biological factors include addressing the inflammation and restoring a healthy periodontium around the implant, similar to the treatment of periodontitis in natural teeth. The proposed treatment protocol involves performing a surgical treatment after non-surgical treatment such as oral hygiene instructions, mechanical cleansing of the fixture, and general or topical antiseptic or antibiotic application according to the extent of inflammation.
5 The choice of surgical treatment is based on the shape of the surrounding bone defect, and the surgical options are resective and regenerative surgery. To date, a definite treatment protocol for peri-implantitis has not been established, despite several proposals.
We would like to present a 6-year follow-up case showing unusual marginal bone regeneration after resective surgery and decontamination of an implant surface for the treatment of peri-implantitis and discuss the possible reasons.
Go to :

Case Report
A 59-year-old male patient was admitted to the Department of Periodontology of Dental Hospital with the chief complaint of discomfort at the right mandibular second premolar implant (i45) in 2015. The patient had no remarkable systemic disease affecting the dental condition. I45 was installed in a local dental clinic 13 years ago. A clinical examination conducted on the i45 showed midbuccal 7-mm probing depth (PD), bleeding on probing (BOP, +), and pus discharge. The radiographic examination confirmed supporting bone loss (≥ 2 mm) of i45, and peri-implantitis was diagnosed (
Fig. 1).
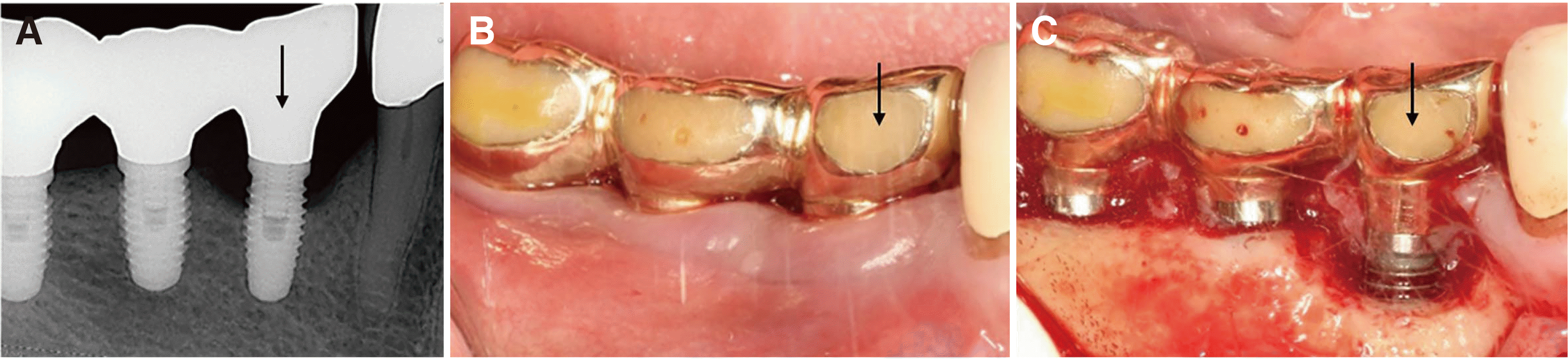 | Fig. 1The initial clinical and radiographic examination. Note the marginal bone loss of approximately 1/3 of the total fixture length of i45 (A) and marginal gingival recession and bleeding tendency (B). Flap elevation and debridement of i45 during resective surgery (C). Note the marginal bone loss and the exposed fixture thread of i45 whereas the surrounding bone is thick and intact around i46 and i47. 
|
A treatment plan was established based on cumulative interceptive supportive therapy (CIST), a step-by-step treatment protocol for peri-implantitis proposed by Mombelli and Lang.
5 In this patient, the following were noted: PD > 5 mm, BOP (+), bone loss > 2 mm, and horizontal bone defect. The treatment plan was as follows: mechanical debridement (A) + application of a local antiseptic (B) + systemic antimicrobial therapy (C)+ resective surgery (D).
First, the patient was re-instructed on oral hygiene and motivated to initiate and continue maintenance. Mechanical debridement was performed using titanium curettes around i45. Chemical plaque control was performed using 0.12% chlorhexidine digluconate (CHX; Hexamedine®, Bukwang Pharm, Seoul, Korea), as mouth rinses twice daily, and intrasulcular antiseptic irrigation using CHX. And topical application of minocycline HCl gel (Minocline®, Dongkook Pharma, Seoul, Korea) as a local antibiotic therapy once a week for 2 weeks. After the non-surgical modalities were completed, the periodontal deep pocket remained, and pus was observed upon gentle pressure to i45.
Therefore, surgical treatment was planned to clean the implant surface and reduce peri-implant pocket depth. Since the shape of the bone defect appeared to be a horizontal defect, resective surgery was planned instead of a regenerative approach.
After administering the appropriate local anesthesia, a mucoperiosteal full-thickness flap was elevated and the site was thoroughly curetted. A 5-mm horizontal suprabony defect was observed and mechanical cleansing of i45 surface was performed using titanium curettes. Alveoloplasty was performed to remove the buccal and lingual ledge-shaped marginal bone using a low-speed round bur to recover the physiological ridge contour. Decontamination of the implant surface was performed by rubbing the surface with cotton soaked in CHX and washing with normal saline solution repeatedly. The flap was sutured without any attempt for bone regeneration. CHX mouthwash and general antibiotics were prescribed for 10 days according to the CIST protocol. Unfortunately, after suture removal and 3-week follow-up, the patient did not visit our clinic for 5 years.
In June, 2020, the patient revisited our clinic again after 5 years because of pain in i46. Peri-implantitis was also diagnosed in i46 through clinical and radiographic examinations. We confirmed marginal bone regeneration around i45, which was treated 5 years ago, by radiographic screening; a second resective surgery was planned for the treatment of i46 (
Fig. 2).
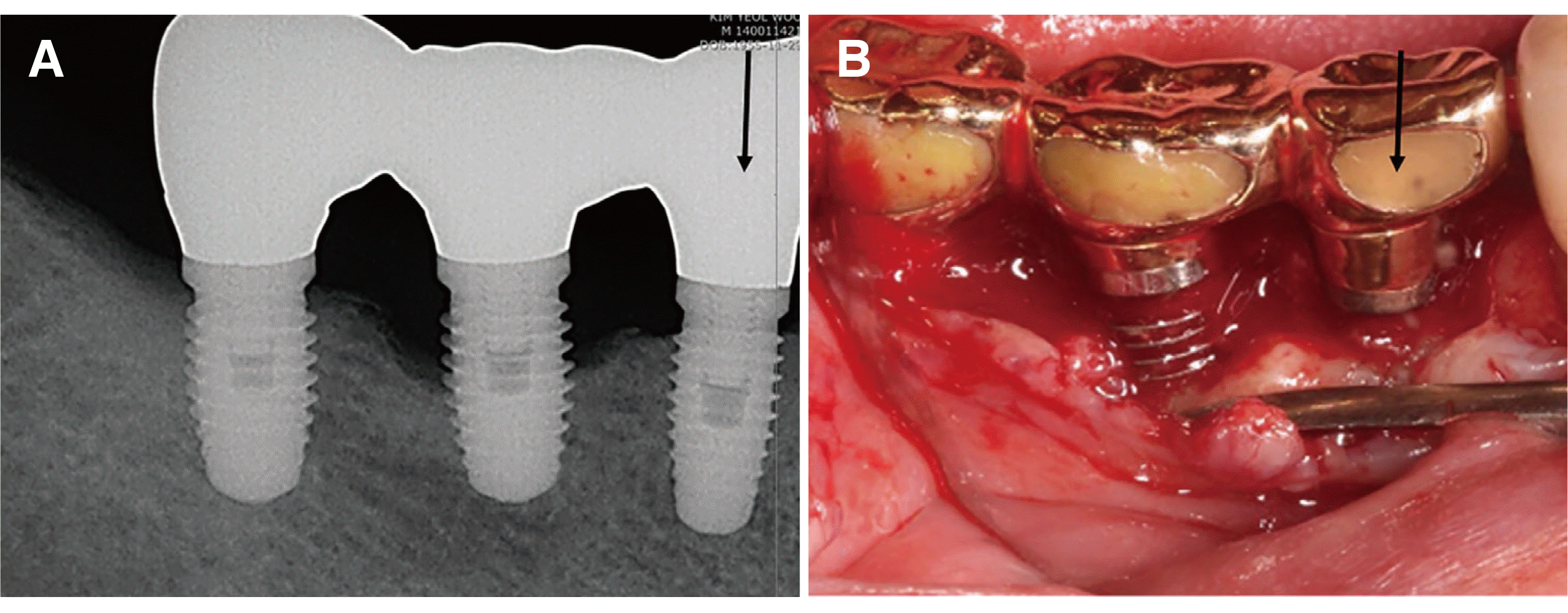 | Fig. 2Radiograph and clinical photo 5 years after resective surgery. Note the regeneration of the crestal bone around i45 and occurrence of peri-implantitis of i46 (A). Severe marginal loss of i46 and bone regeneration of i45 are observed during the second resective surgery for the treatment of i46 (B). 
|
Resective surgery of i46 was performed in the same way as the resective surgery of i45, 5 years prior. After two resective surgeries for the treatment of peri-implantitis on the same adjacent implants, stable shallow probing depth, BOP (-), and pus (-) were observed on clinical examination, indicating that the periodontium around i45 and i46 had recovered.
The patient had no discomfort, and good healing was observed on radiographic and clinical examinations 6 years after the first resective surgery of i45 and 1 year after the resective surgery of i46 for the treatment of peri-implantitis (
Fig. 3).
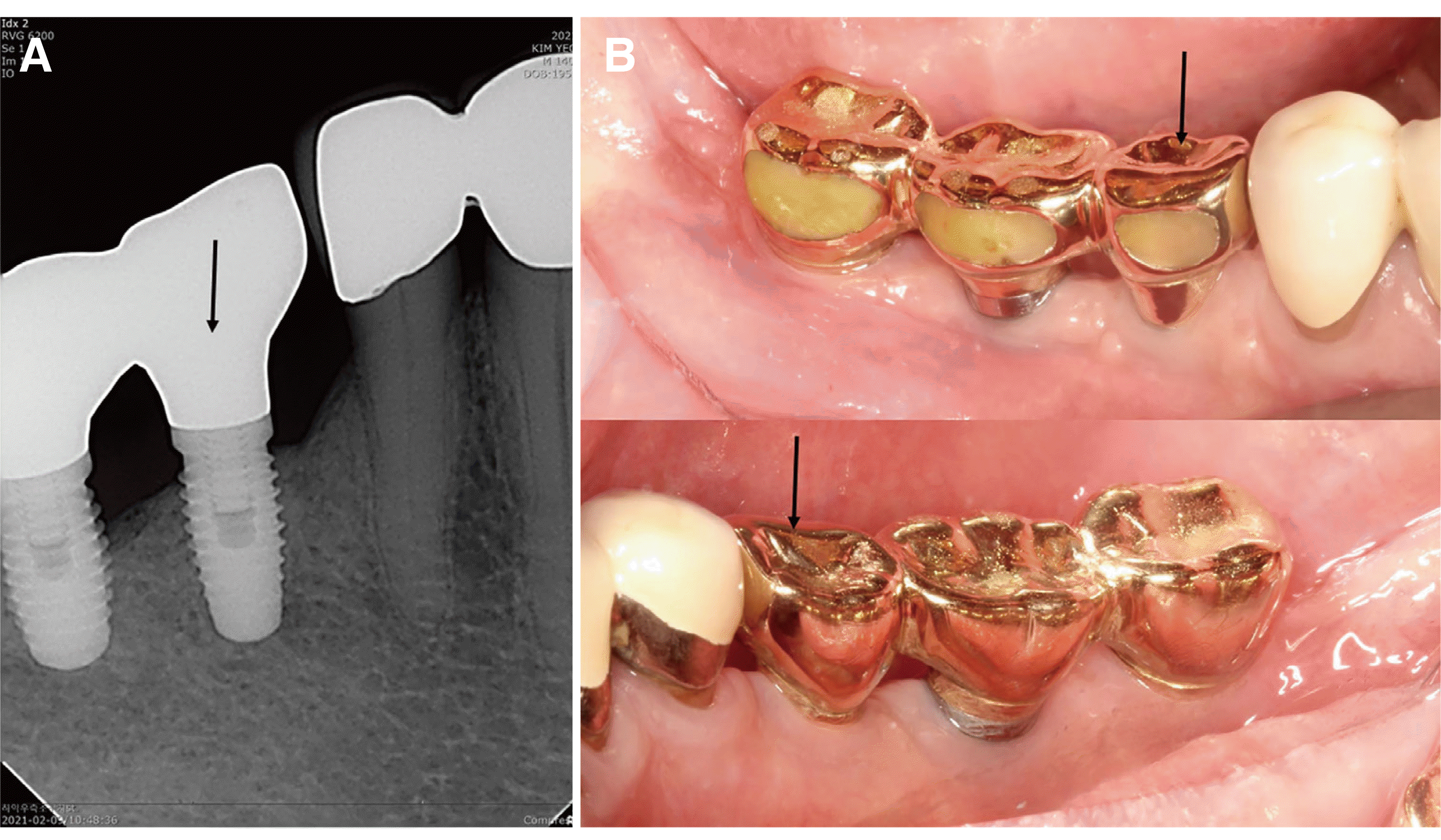 | Fig. 3Radiograph and clinical photos taken 6 years after the 1st resective surgery and 1 year after the 2nd resective surgery. Note the crestal bone regeneration of i45, stable crestal bone level of i46 (A), and the healthy gingival color and shape despite mild gingival recession (B). 
|
Go to :

Discussion
Treatment methods of peri-implantitis are categorized into non-surgical or surgical method. Non-surgical methods are essential in preparing healthy periodontal tissue before surgical procedure. Especially, the removal of the bacterial biofilm of the exposed fixture is mandatory for restoring gingival health. Many studies have suggested various implant surface decontamination methods; air-abrasive instrument with glycin,
6 irradiation with and Er:YAG laser,
7 washing with 0.12% CHX and saline
8 and so on. Since there is no absolutely superior method among the currently reported methods, simple and effective method was adopted in this case.
Six years after the resective surgery wherein peri-implantitis of i45 was diagnosed, buccal and lingual marginal bone regeneration of approximately 3 mm (3 threads of fixture) and absence of inflammation in the treated area were observed. This unusual crestal bone regeneration was unexpected and uncommon. Our initial treatment goal was to reduce the probing depth through the resolution of inflammation, without grafting or any other regenerative approach, since the form of the defect, i.e., the marginal horizontal defect, was not favorable for regenerative surgery. There was no intermediate follow-up; however, buccal and lingual bone regeneration occurred 6 years later. Regeneration mechanisms can be considered in various ways.
The primary factors for successful regeneration of osseous defects proposed by Kornman and Robertson
9 are as follows: 1) bacterial contamination, 2) innate wound healing potential, 3) local site characteristics, and 4) surgical procedure. To control bacterial contamination, mechanical non-surgical therapy was performed before the surgical procedure based on CIST.
5 At the first visit, the patient’s oral hygiene level was favorable, except for i45, which had BOP (+) and pus discharge. In addition to the patient’s good oral hygiene care, the implant surface was scaled and polished using instruments during the non-surgical treatment phase and the area was rinsed twice daily with 0.12% CHX. During the surgical phase, active decontamination around the implant surface was attempted using cotton balls soaked in CHX solution and saline.
It can be assumed that the preserved periosteum has innate wound healing potential. The periosteum is a well-vascularized osteogenic organ with structures that contain capillaries, osteoblasts, and mesenchymal stem cells. The interaction between peripheral factors involved in bone healing, mesenchymal stem cells of the periosteum, and the preservation of blood supply increases the healing potential.
10 The highly abundant growth factors derived from platelets can stimulate the proliferation and differentiation of mesenchymal stem cells of the periosteum. Spontaneous self-regeneration of the mandible following large resective surgeries has been reported, in which the periosteum seems to play a major role.
11,12 The blood supply can be better maintained, and healing potential can be promoted if the periosteum is preserved without tearing during surgery.
13 It can also be seen that the periosteum acts as a natural membrane that replaces the artificial membrane.
14 It is known that the activation of periosteal-derived progenitor cells induces strong cartilage and bone formation, accompanied by a remarkable induction of angiogenesis, and ultimately induces vascularization and remodeling of bone grafts.
Next, the shape and amount of bone defect is considered as another factors. Botticelli et al.
15,16 showed that the resolution of marginal four-wall defects within a “jumping distance” around the implant surface without graft materials and the amount of regeneration seems to be dependent on both the defect size and healing time in a canine model. Moreover, buccal open marginal defects were partially restored after 4 months of healing without graft materials.
17 In our case, a 5-mm marginal bone loss was observed in the thick periodontal biotype (thick gingiva, periosteum, and thick bone-thickness). Defect configuration and healing potential of the surrounding tissues are favorable for spontaneous bone regeneration. Tawil and Tawil
2 also reported unusual bone regeneration of implants with peri-implantitis; the regenerative process progressed steadily at a rate of 1 to 1.5 mm per year and was completed at 3 years with no clinical intervention besides regular oral hygiene. They also stressed the importance of the periosteum in bone regeneration. The exceptional healing obtained and the time needed to reach full regeneration of the defect, relying exclusively on the individual healing potential in the absence of any graft, are worthy of further investigation.
Finally, the surgical procedures can be another factor for regeneration. The operator’s skill and proficiency, which involves the selection of surgical treatment options and management of the tissue, such as atraumatic flap management and preservation of the periosteum, may also have affected the healing potential.
Go to :

Conclusion
According to our presentation, the mechanism and predictability of bone regeneration are not completely understood. From a clinical perspective, resective surgery with implant surface decontamination is a very effective protocol for peri-implantitis with horizontal marginal bone loss. Regeneration potential can be enhanced by the interaction of various factors such as healthy periosteum, sufficient native bone volume, thick bone biotype, meticulous decontamination of the implant surface, and the operator’s proficiency.
Go to :

Acknowledgements
This work was supported by a 2-Year Research Grant of Pusan National University.
Go to :

References
1. Derks J, Schaller D, Håkansson J, Wennström JL, Tomasi C, Berglundh T. 2016; Effectiveness of implant therapy analyzed in a Swedish population: Prevalence of peri-implantitis. J Dent Res. 95:43–9. DOI:
10.1177/0022034515608832. PMID:
26701919.

2. Tawil G, Tawil P. 2019; Peri-implant infection concomitant with a flare-up episode of chronic periodontitis: An unusual regeneration following treatment and a 5-year follow-up. Int J Periodontics Restorative Dent. 39:415–21. DOI:
10.11607/prd.3940. PMID:
30986291.

3. Cho-Yan Lee J, Mattheos N, Nixon KC, Ivanovski S. 2012; Residual periodontal pockets are a risk indicator for peri-implantitis in patients treated for periodontitis. Clin Oral Implants Res. 23:325–33. DOI:
10.1111/j.1600-0501.2011.02264.x. PMID:
22092508.

6. Singh G, O'Neal RB, Brennan WA, Strong SL, Horner JA, Van Dyke TE. 1993; Surgical treatment of induced peri-implantitis in the micro pig: clinical and histological analysis. J Periodontol. 64:984–9. DOI:
10.1902/jop.1993.64.10.984. PMID:
8277409.

7. Kreisler M, Kohnen W, Christoffers AB, Götz H, Jansen B, Duschner H, d'Hoedt B. 2005; In vitro evaluation of the biocompatibility of contaminated implant surfaces treated with an Er : YAG laser and an air powder system. Clin Oral Implants Res. 16:36–43. DOI:
10.1111/j.1600-0501.2004.01056.x. PMID:
15642029.

8. Wetzel AC, Vlassis J, Caffesse RG, Hämmerle CH, Lang NP. 1999; Attempts to obtain re-osseointegration following experimental peri-implantitis in dogs. Clin Oral Implants Res. 10:111–9. DOI:
10.1034/j.1600-0501.1999.100205.x. PMID:
10219130.

11. Sharma P, Williams R, Monaghan A. 2013; Spontaneous mandibular regeneration: another option for mandibular reconstruction in children? Br J Oral Maxillofac Surg. 51:e63–6. DOI:
10.1016/j.bjoms.2012.04.255. PMID:
22578705.

12. Ahmad O, Omami G. 2015; Self-regeneration of the mandible following hemimandibulectomy for ameloblastoma: a case report and review of literature. J Maxillofac Oral Surg. 14(Suppl 1):245–50. DOI:
10.1007/s12663-012-0462-7. PMID:
25861189. PMCID:
PMC4379248.

13. Verdugo F, Simonian K, D'Addona A, Pontón J, Nowzari H. 2010; Human bone repair after mandibular symphysis block harvesting: A clinical and tomographic study. J Periodontol. 81:702–9. DOI:
10.1902/jop.2010.090612. PMID:
20429649.

14. Verdugo F, D'Addona A, Pontón J. 2012; Clinical, tomographic, and histological assessment of periosteal guided bone regeneration with cortical perforations in advanced human critical size defects. Clin Implant Dent Relat Res. 14:112–20. DOI:
10.1111/j.1708-8208.2009.00235.x. PMID:
20491815.

15. Botticelli D, Berglundh T, Buser D, Lindhe J. 2003; The jumping distance revisited: An experimental study in the dog. Clin Oral Implants Res. 14:35–42. DOI:
10.1034/j.1600-0501.2003.140105.x. PMID:
12562363.
17. Botticelli D, Berglundh T, Lindhe J. 2004; Resolution of bone defects of varying dimension and configuration in the marginal portion of the peri-implant bone. An experimental study in the dog. J Clin Periodontol. 31:309–17. DOI:
10.1111/j.1600-051X.2004.00502.x. PMID:
15016260.

Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download