Abstract
Background
Recent American guideline published in 2020 recommend using 24-h area under the curve (AUC)/minimum inhibitory concentration instead of vancomycin serum concentration (Ctrough) for vancomycin therapeutic drug monitoring (TDM). However, Ctrough-based TDM is widely used in clinical practice. Thus, this retrospective study aimed to compare Ctrough-based and AUC-based TDM.
Methods
We evaluated patients’ TDM data with at least one vancomycin trough measurement. Patients younger than 18 years, admitted to an intensive care unit, or on renal replacement therapy were excluded. The variables of Ctrough-based and AUC-based TDM were simulated using MwPharm++ (Mediware, Czech Republic) with vancomycin two-compartment model. The therapeutic range was 400-600 mg*h/L and 15-20 mg/L for AUC and Ctrough, respectively. We evaluated the correlation between Ctrough and AUC, the attainment rate of AUC target range, and changes in vancomycin dose and Ctrough when AUC-based TDM is applied.
Results
One hundred and four patients were enrolled. Ctrough and AUC correlated moderately (R=0.707, P<0.001). Among 31 patients with Ctrough of 15-20 mg/L, the AUC of only 18 patients was within the target range (18/31, 58.1%). In addition, most patients with Ctrough of 10-15 mg/L had the AUC within the target range (57/66, 86.4%). The respective vancomycin dose and Ctrough were expected to be significantly lower in AUC-based TDM simulation than those in Ctrough-based TDM simulation
초록
배경
2020년 발표된 미국 가이드라인에서는 반코마이신 치료약물농도감시(TDM)에 최저농도 대신 24시간 곡선아래면적(AUC)/최소억제농도를 이용할 것을 권고하였다. 하지만 아직도 실제 임상 환경에서는 최저농도 기반 TDM이 보편적으로 이용되고 있는 실정이다. 본 후향적 연구에서는 최저농도 기반 TDM과 AUC 기반 TDM을 비교평가 하고자 하였다.
방법
1회 이상 반코마이신 최저농도를 측정한 환자들의 TDM 자료를 분석하였다. 18세 미만 환자와 중환자실 입원 환자, 신대체요법을 받고 있는 환자는 대상자에서 제외하였다. 최저농도 기반 TDM과 AUC 기반 TDM 시뮬레이션에는 MwPharm++ (Mediware, Czech Republic) 반코마이신 2 구획 모델을 이용하였다. AUC 및 최저농도의 치료목표범위는 각각 400-600 mg*h/L 및 15-20 mg/L로 정의하였다. 예측된 최저농도와 AUC의 상관관계, AUC 치료범위 달성률, 최저농도 대신 AUC 기반 TDM을 채택하였을 때의 반코마이신 용량 및 최저농도 변화를 분석하였다.
Vancomycin is a glycopeptide antibiotic that inhibits bacterial cell wall synthesis by binding to the D-alanyl-D-alanine terminus of cell-wall precursors [1]. Due to its excellent antibiotic effect against gram-positive bacteria, vancomycin is used as the first-line therapy for methicillin-resistant Staphylococcus aureus (MRSA) infection, and other various indications including neutropenic fever, coagulase negative staphylococcus (CoNS), and other gram-positive bacterial infections [1]. However, since vancomycin has a narrow therapeutic index, therapeutic drug monitoring (TDM) is necessary for improving efficacy while preventing possible side effects, especially nephrotoxicity [2].
Since the release of the first consensus guideline on vancomycin monitoring, the 24-h AUC (AUC)/minimum inhibitory concentration (MIC) has been the primary pharmacokinetic (PK)/pharmacodynamic (PD) index. Nevertheless, serum vancomycin trough concentration (Ctrough) has been suggested to be a surrogate monitoring index because it is easily available [2, 3]. However, the increase in vancomycin PK data raised concerns regarding Ctrough use since Ctrough greater than 15 mg/L frequently caused high AUC, which was associated with frequent vancomycin toxicity. Vancomycin TDM guidelines have been changed to reflect these new findings. The American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), the Pediatric Infectious Disease Society (PIDS), and the Society of Infectious Disease Pharmacists (SIDP) published a new consensus guideline in 2020 suggesting the use of AUC/MIC instead of Ctrough for vancomycin monitoring in patients with serious MRSA infection [4]. Moreover, the revised guideline of the Chinese Pharmacological Society included the recommendation for monitoring AUC along with Ctrough for vancomycin TDM [5].
However, due to the many drawbacks of AUC-based TDM, particularly the difficulty in obtaining AUC and unfamiliarity, many clinicians are still using Ctrough for vancomycin monitoring. Surveys on the implementation and perceptions of vancomycin monitoring revealed that significant number of pharmacists, physicians, and institutions were unaware of the transition to AUC-based TDM or were not planning to adopt AUC-based TDM [6-9]. Therefore, intuitive investigation of the relationships between AUC and Ctrough and the AUC attainment rate of Ctrough-based TDM would help physicians use the adjusted TDM target in clinical practice.
This retrospective in silico study analyzed previous TDM data to assess the correlation between Ctrough and AUC, the attainment rate of AUC target range, and the change in vancomycin dose based on a recent American guideline and the commonly used Ctrough-based TDM in a Korean population.
We evaluated the TDM data of patients who were referred for TDM consultation from April 1st, 2019, to April 30th, 2020, at the Department of Laboratory Medicine and Genetics, Samsung Medical Center Seoul, Korea. Patients with at least one vancomycin trough measurement were enrolled. Patients who were younger than 18 years, admitted to an intensive care unit, or on renal replacement therapy were excluded. If patients were consulted multiple times during the given period, data from first TDM consultation were used for analysis. For impact analysis when transitioning to AUC-based TDM from trough-based TDM, Ctrough data within 15-20 mg/L with the given regimen were included.
The following demographic characteristics, clinical information, and laboratory data were obtained for analysis and TDM: sex, age, height, body weight, diagnosis, vancomycin indication, vancomycin regimen, sampling time, serum vancomycin concentration, and serum creatinine. Creatinine clearance (CLCr) was calculated from serum creatinine concentration using Cockcroft-Gault equation. Vancomycin concentration was measured by the kinetic interaction of microparticles in solution immunoassay on Roche Cobas c702 analyzer (Roche, Basel, Switzerland). Based on our institution protocol, initial vancomycin measurements were performed prior to the third or fourth vancomycin injection. Follow-up vancomycin measurements, mostly prior to the third injection, were performed at the physician’s discretion.
We used target AUC and Ctrough ranges recommended by the American guidelines. The primary Ctrough target range was established based on vancomycin indication: 15-20 mg/L for bacteremia, sepsis, pneumonia, catheter-related blood stream infection (CLABSI), central nervous system infection, and endocarditis, and 10-15 mg/L for neutropenic fever, skin infection, post-operative wound infection, otitis, and other non-complicated infections. The therapeutic range for AUC was 400-600 mg*h/L for serious MRSA infection and was extrapolated to other indications to explore the PK dynamics of vancomycin. The AUC attainment rate was defined as the proportion of patients with AUC within 400-600 mg*h/L divided by the total patients in the given Ctrough target range. Similarly, the rate of failure to obtain AUC therapeutic range (failure rate) was defined as the proportion of patients who did not have AUC within the therapeutic range divided by the total patients in the given Ctrough target range.
Estimated Ctrough and AUC were calculated with commercial Bay-esian PK software, MWPharm++ (MW) (Mediware, Praha, Czech Republic), using two-compartment vancomycin KKGT (Dutch Association for Quality Assessment in TDM and Clinical Toxicology) population model [10]. The mean and standard deviation of PK fitting parameter were as follows: C01.V (L/KgLBMc)=0.21±0.04, C02.kxy (1/h)=1.12±0.28, C02.kyx (1/h)=0.48±0.12, and RE.k (1/h/(mL/min/1.73 m2))=0.00327±0.00109. Patient covariates were age, sex, height, weight, and CLCr. For each patient, the recommended regimen for previous Ctrough-based TDM consultations was simulated. The correlation between Ctrough and AUC was evaluated, and the rate of failure to achieve AUC therapeutic range was calculated for Ctrough target range with lower limit of 0-20 mg/L and range interval of 3.0 mg/L, 3.5 mg/L, 4.0 mg/L, 4.5 mg/L, and 5.0 mg/L. Furthermore, to investigate the expected changes in dose and trough when changing monitoring index from Ctrough to AUC, additional analysis was performed on patients with Ctrough of 15-20 mg/L. The doses needed to obtain AUC 400 mg*h/L (AUC400), 500 mg*h/L (AUC500), and 600 mg*h/L (AUC600) and the corresponding Ctrough values were calculated.
MedCalc® version 77.5.1.0 (MedCalc Software Ltd., Ostend, Belgium) and IBM SPSS® Statistics version 25 (IBM Corp., Armonk, NY, USA) were used for statistical analyses. Pearson correlation analysis for estimated Ctrough and AUC data from all enrolled patients was performed. Paired t-test and Wilcoxon signed rank test were performed to demonstrate statistically significant differences between Ctrough and vancomycin dose of AUC-based TDM. A P-value less than 0.05 was considered statistically significant.
This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center, Seoul, Korea (IRB number. SMC 2021-02-125-001), and the need for informed consent was waived.
The detailed characteristics of patients are described in Table 1. Vancomycin TDM data from 104 patients were available for correlation analysis between Ctrough-based and AUC-based TDM. Under the Ctrough-based recommended regimen, estimated Ctrough was categorized into three groups: less than 10 mg/L (n=7), 10-15 mg/L (n=66), and 15-20 mg/L (n=31), as was estimated AUC: less than 400 mg*h/L (n=7), 400-600 mg*h/L (n=79), and 600 mg*h/L or more (n=18).
The AUC was within the therapeutic range in 18 of 31 patients with Ctrough of 15-20 mg/L (18/31; attainment rate, 58.1%). On the other hand, 57 patients with Ctrough of 10-15 mg/L had AUC within the therapeutic range (57/66; attainment rate, 86.4%). These results suggest that the AUC therapeutic range is difficult to obtain with the previously suggested target range of Ctrough. Instead, a lower Ctrough is needed to achieve the AUC therapeutic range. Pearson correlation analysis revealed moderate correlation between estimated Ctrough and AUC (R=0.707, P<0.001) (Fig. 1).
In estimating failure rate when using Ctrough range, lower failure rates were observed at target ranges of 11.9 mg/L-14.9 mg/L, 11.0 mg/L-14.5 mg/, 10.7 mg/L-14.7 mg/L, 10.2 mg/L-14.7 mg/L, and 9.5 mg/L-14.5 mg/L (Table 2). The upper limits of all suggested Ctrough target ranges were near 15 mg/L, and the lower limits varied by range interval. Each candidate Ctrough target range corresponded with AUC therapeutic range in 90% of patients.
Of the 104 patients, 31 met the 15-20 mg/L target based on previous TDM Ctrough target. Among them, data from 28 patients were included for the assessment of change in vancomycin dose and Ctrough when using AUC-based TDM. There were statistically significant decreases in both vancomycin dose and Ctrough when targeting AUC 400 mg*h/L and 500 mg*h/L compared to the values when targeting Ctrough of 15-20 mg/L (Fig. 2).
To the best of our knowledge, this is the first study comparing AUC-based and trough-based TDM using data from real clinical practices in Korean patients. In this retrospective study comparing Ctrough-based TDM and AUC-based TDM, the achievement of target range and the correlation between Ctrough and AUC were assessed in a Korean population. A moderate correlation was observed between AUC and Ctrough. However, a considerable number of patients with Ctrough of 15-20 mg/L were over-treated based on the recent AUC therapeutic range guidelines. Moreover, most patients with lower than recommended Ctrough had an AUC therapeutic range of 400-600 mg*h/L. This unsatisfactory compatibility between Ctrough target range and AUC target range was consistent with previous findings [4, 11-17]. Compared to trough-based TDM, AUC-based TDM was expected to achieve lower vancomycin dose and Ctrough.
For AUC-based TDM, the revised American guideline has shown that lower vancomycin dosage could be adopted to prevent possible adverse effects of vancomycin, such as nephrotoxicity. The current American guideline based on several studies suggests an upper limit of AUC of 600 mg*h/L to prevent nephrotoxicity [4, 11, 18-21], and AUC-guided dosing based on this target range reduced nephrotoxicity without a significant increase in treatment failure compared to Ctrough-based dosing [15, 20, 22-26]. Based on currently published data, adopting AUC-based TDM would help prevent unnecessary risk of nephrotoxicity.
However, not all institutions have access to Bayesian PK software, and some clinicians still use Ctrough for vancomycin monitoring. Indeed, AUC is not intuitive as Ctrough because it requires additional PK parameters and is derived from the calculation of such parameters. In the most recent survey conducted in 2020, 70% of responding clinicians answered that they had not implemented AUC-based TDM and 43.0% were not planning to adopt AUC-based TDM [6]. Therefore, although AUC-based TDM appears to be superior to trough-based TDM, alternative methods are needed to avoid the hesitancy associated with adopting AUC-based TDM. One option could be establishing an individual target Ctrough based on the initial AUC calculation using the first-order PK equation and monitoring according to an established target Ctrough range until significant conditional change occurs [27]. Further practical assessment and adjustment of vancomycin monitoring would be helpful.
Although this article mainly focused on the American guideline, the Chinese guideline does not discourage the use of Ctrough for monitoring vancomycin. This might be because the Chinese guideline recommends a lower vancomycin Ctrough lower limit (10 mg/L) and higher AUC upper limit (650 mg*h/L) for serious MRSA infections compared to that recommended by the American guideline [3-5]. In this study, to assess whether simply lowering target Ctrough is acceptable and explore the optimal target for Ctrough range, we evaluated the rate of AUC therapeutic range attainment with lower vancomycin Ctrough target range. Regardless of range interval, the upper limits of the optimal candidate ranges were near 15 mg/L, which is lower than the Ctrough suggested by the 2009 American guideline. Considering this low failure rate, using lower target Ctrough would be acceptable when AUC-based TDM is not conducted. However, further study is needed to determine if a low Ctrough target yields the same clinical outcome as AUC-based TDM.
There are some limitations in this study. First, as this study was an in silico prediction, the comparison of clinical outcomes by TDM strategy was not performed. Moreover, when performing TDM simulation, we included all previous TDM consultation data including those with only one vancomycin measurement or non-steady state serum vancomycin concentration and real steady state Ctrough could not be obtained. However, as stated in the current guideline and other studies, vancomycin TDM with the Bayesian approach can yield reliable prediction even with single non-steady state concentration [4, 28]. In addition, by simulating TDM as it is applied currently in practice, the data reflect real-world practice. Finally, this study was based on the assumption of AUC 400-600 mg*h/L as the accepted target range and assessed only PK factors affecting vancomycin administration. The actual applicability of AUC for indications other than serious MRSA infection, such as vancomycin-susceptible Enterococcus infection, was not addressed in this study. A further large-scale prospective study on the extrapolation of AUC target range is needed.
In conclusion, targeting Ctrough of 15-20 mg/L is not appropriate for attaining the target AUC range and is associated with high AUC values. Using AUC-based TDM could result in a lower vancomycin dose. Applying a low Ctrough might be acceptable, but further large study on the actual clinical outcomes is needed.
Acknowledgements
This study is based on the work supported by the Ministry of Trade, Industry & Energy (MOTIE, Korea) under Industrial Technology Innovation Program [No.10080648].
REFERENCES
1. Blaskovich MAT, Hansford KA, Butler MS, Jia Z, Mark AE, Cooper MA. 2018; Developments in glycopeptide antibiotics. ACS Infect Dis. 4:715–35. DOI: 10.1021/acsinfecdis.7b00258. PMID: 29363950. PMCID: PMC5952257.

2. Rybak MJ. 2006; The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 42(S1):S35–9. DOI: 10.1086/491712. PMID: 16323118.

3. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, et al. 2009; Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 29:1275–9. DOI: 10.1592/phco.29.11.1275. PMID: 19873687.
4. Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. 2020; Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 77:835–64. DOI: 10.1093/ajhp/zxaa036. PMID: 32191793.
5. He N, Su S, Ye Z, Du G, He B, Li D, et al. 2020; Evidence-based guideline for therapeutic drug monitoring of vancomycin: 2020 update by the division of therapeutic drug monitoring, Chinese Pharmacological Society. Clin Infect Dis. 71(S4):S363–71.

6. Bradley N, Lee Y, Sadeia M. 2021; Assessment of the implementation of AUC dosing and monitoring practices with vancomycin at hospitals across the United States. J Pharm Pract. 8971900211012395. DOI: 10.1177/08971900211012395. PMID: 33902351.

7. Gregory ER, Burgess DR, Cotner SE, VanHoose JD, Flannery AH, Gardner B, et al. 2021; Pharmacist survey: Pharmacist perception of vancomycin area under the curve therapeutic drug monitoring. J Pharm Pract. 34:272–8. DOI: 10.1177/0897190019867494. PMID: 31422738.

8. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. 2019; Readiness to implement vancomycin monitoring based on area under the concentration-time curve: A cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 76:889–94. DOI: 10.1093/ajhp/zxz070. PMID: 31063582.

9. Flannery AH, Hammond DA, Oyler DR, Li C, Wong A, Smith AP, et al. 2020; Vancomycin dosing practices among critical care pharmacists: A survey of Society of Critical Care Medicine Pharmacists. Infect Dis (Auckl). 13:1178633720952078. DOI: 10.1177/1178633720952078. PMID: 33029073. PMCID: PMC7522823.

10. Neef C, Touw DJ, Harteveld AR, Eerland JJ, Uges DR. 2006; Pitfalls in TDM of antibiotic drugs: Analytical and modelling issues. Ther Drug Monit. 28:686–9. DOI: 10.1097/01.ftd.0000243966.97964.11. PMID: 17038886.

11. Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. 2014; Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 58:309–16. DOI: 10.1128/AAC.01653-13. PMID: 24165176. PMCID: PMC3910745.

12. Hale CM, Seabury RW, Steele JM, Darko W, Miller CD. 2017; Are vancomycin trough concentrations of 15 to 20 mg/L associated with increased attainment of an AUC/MIC ≥ 400 in patients with presumed MRSA infection? J Pharm Pract. 30:329–35. DOI: 10.1177/0897190016642692. PMID: 27074786.

13. Clark L, Skrupky LP, Servais R, Brummitt CF, Dilworth TJ. 2019; Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented staphylococcal infections. Ther Drug Monit. 41:483–8. DOI: 10.1097/FTD.0000000000000622. PMID: 30817704.

14. Pai MP, Neely M, Rodvold KA, Lodise TP. 2014; Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 77:50–7. DOI: 10.1016/j.addr.2014.05.016. PMID: 24910345.

15. Marko R, Hajjar J, Nzeribe V, Pittman M, Deslandes V, Sant N, et al. 2021; Therapeutic drug monitoring of vancomycin in adult patients with methicillin-resistant Staphylococcus aureus bacteremia or pneumonia. Can J Hosp Pharm. 74:334–43. DOI: 10.4212/cjhp.v74i4.3195. PMID: 34602621. PMCID: PMC8463016.
16. AbuSara AK, Abdelrahman DH, Habash KI, Al-Shaer MH, Le J, Nazer LH. 2022; Vancomycin therapeutic monitoring by measured trough concentration versus Bayesian-derived area under the curve in critically ill patients with cancer. Pharmacol Res Perspect. 10:e00912. DOI: 10.1002/prp2.912. PMID: 34990089. PMCID: PMC8929348.

17. Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. 2011; Vancomycin: We can't get there from here. Clin Infect Dis. 52:969–74. DOI: 10.1093/cid/cir078. PMID: 21460308.

18. Al-Sulaiti FK, Nader AM, Saad MO, Shaukat A, Parakadavathu R, Elzu-bair A, et al. 2019; Clinical and pharmacokinetic outcomes of peak-trough-based versus trough-based vancomycin therapeutic drug monitoring approaches: A pragmatic randomized controlled trial. Eur J Drug Metab Pharmacokinet. 44:639–52. DOI: 10.1007/s13318-019-00551-1. PMID: 30919233. PMCID: PMC6746691.

19. Suzuki A, Hamada Y, Ikeda H, Tanaka H, Yanagihara M, Namiki M, et al. 2021; Comparison of trough concentration and area under the curve of vancomycin associated with the incidence of nephrotoxicity and predictors of a high trough level. J Infect Chemother. 27:455–60. DOI: 10.1016/j.jiac.2020.10.014. PMID: 33144145.

20. Mogle BT, Steele JM, Seabury RW, Dang UJ, Kufel WD. 2018; Implementation of a two-point pharmacokinetic AUC-based vancomycin therapeutic drug monitoring approach in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 52:805–10. DOI: 10.1016/j.ijantimicag.2018.08.024. PMID: 30176357.
21. Liu J, Tong SYC, Davis JS, Rhodes NJ, Scheetz MH. 2020; Vancomycin exposure and acute kidney injury outcome: A snapshot from the CAMERA2 study. Open Forum Infect Dis. 7:ofaa538. DOI: 10.1093/ofid/ofaa538. PMID: 33335938. PMCID: PMC7731530.

22. Lines J, Burchette J, Kullab SM, Lewis P. 2021; Evaluation of a trough-only extrapolated area under the curve vancomycin dosing method on clinical outcomes. Int J Clin Pharm. 43:263–9. DOI: 10.1007/s11096-020-01157-3. PMID: 32964405.

23. Rees MR, Carr DR, Trienski T, Buchanan C, White K, Bremmer DN. 2022; Outpatient vancomycin therapy: Acute kidney injury in individualized AUC-based goal trough ranges versus traditional trough dosing. J Am Pharm Assoc (2003). 62:706–10. DOI: 10.1016/j.japh.2021.11.031. PMID: 34920955.

24. Oda K, Jono H, Nosaka K, Saito H. 2020; Reduced nephrotoxicity with vancomycin therapeutic drug monitoring guided by area under the concentration-time curve against a trough 15-20 μg/mL concentration. Int J Antimicrob Agents. 56:106109. DOI: 10.1016/j.ijantimicag.2020.106109. PMID: 32721597.

25. Katip W, Okonogi S, Oberdorfer P. 2022; The thirty-day mortality rate and nephrotoxicity associated with trough serum vancomycin concentrations during treatment of enterococcal infections: A propensity score matching analysis. Front Pharmacol. 12:773994. DOI: 10.3389/fphar.2021.773994. PMID: 35153743. PMCID: PMC8831381.

26. Ueda T, Takesue Y, Nakajima K, Ichiki K, Ishikawa K, Yamada K, et al. 2022; Validation of vancomycin area under the concentration-time curve estimation by the Bayesian approach using one-point samples for predicting clinical outcomes in patients with methicillin-resistant Staphylococcus aureus infections. Antibiotics (Basel). 11:96. DOI: 10.3390/antibiotics11010096. PMID: 35052972. PMCID: PMC8772855.

27. Nix DE, Davis LE, Matthias KR. 2022; The relationship of vancomycin 24-hour AUC and trough concentration. Am J Health Syst Pharm. 79:534–9. DOI: 10.1093/ajhp/zxab457. PMID: 34849533.

28. Jager NGL, Chai MG, van Hest RM, Lipman J, Roberts JA, Cotta MO. Precision dosing software to optimise antimicrobial dosing: a systematic search and follow-up survey of available programs. Clin Microbiol Infect. 2022; doi: 10.1016/j.cmi.2022.03.041 (in press). DOI: 10.1016/j.cmi.2022.03.041. PMID: 35429656.
Fig. 1
Correlation between estimated Ctrough and AUC (N=104). Regression line with 95% CI is shown.
Abbreviations: Ctrough, serum vancomycin trough concentration; AUC, area under the curve for 24 h; CI, confidence interval.
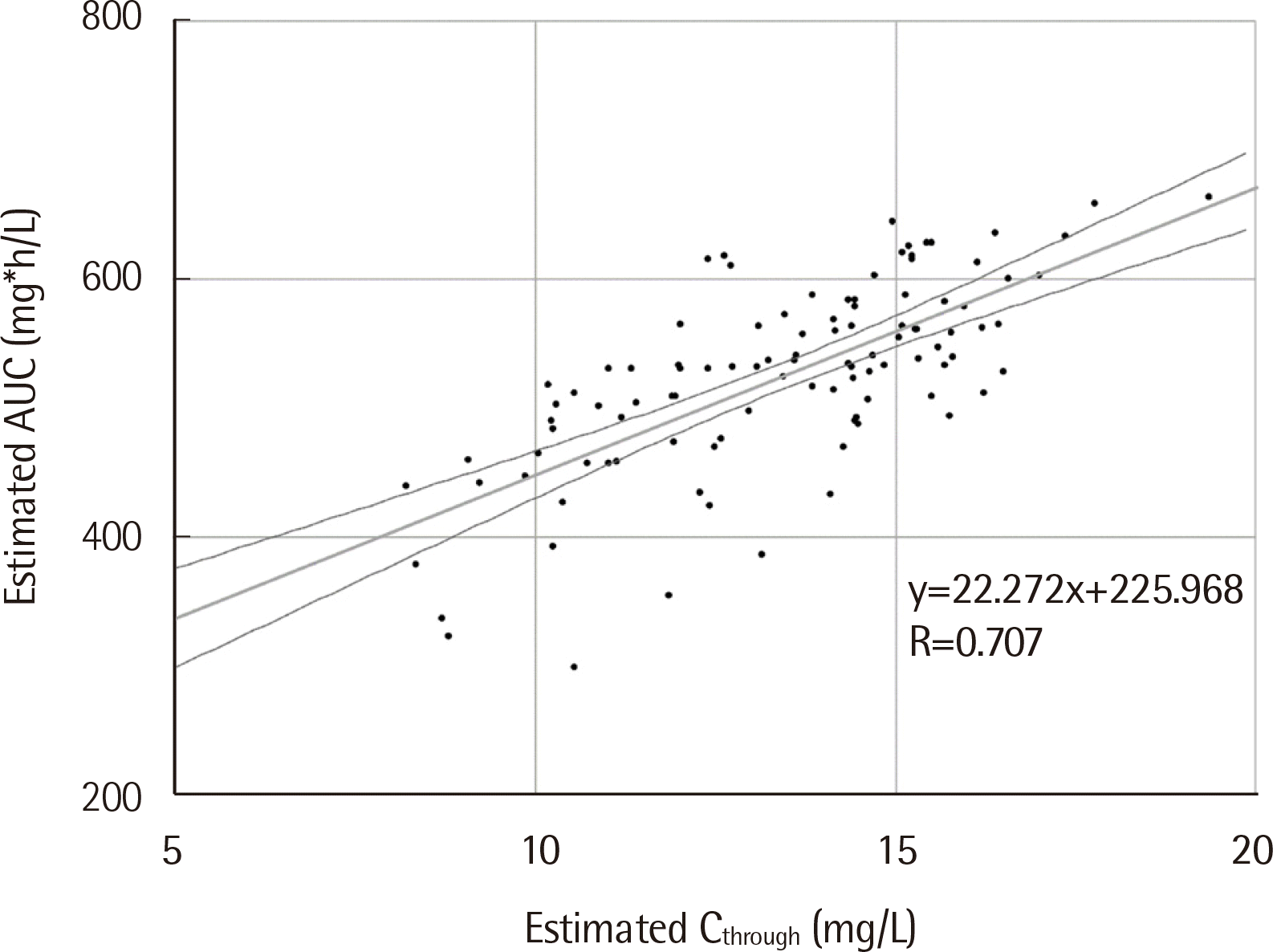
Fig. 2
Comparison of (A) vancomycin dosage per kg and (B) serum vancomycin trough concentration by monitoring target (N=28). The monitoring target is defined as follows: Ctrough, serum vancomycin trough concentration of 15-20 mg/L; AUC400, AUC/MIC 400; AUC500, AUC/MIC 500; and AUC600, AUC/MIC 600, with MIC assumed to be 1 mg/L.
Abbreviations: AUC, area under the curve for 24 h; MIC, minimum inhibitory concentration; Ctrough, serum vancomycin trough concentration.
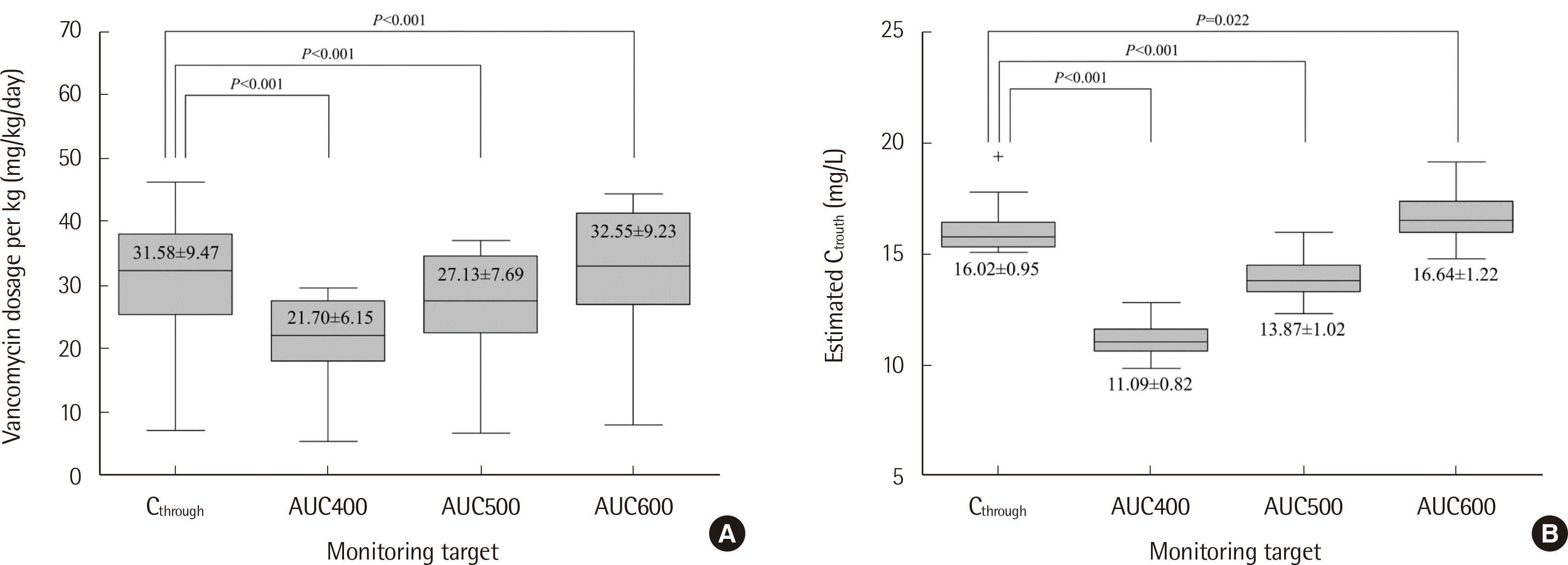
Table 1
Patient characteristics
| Parameter | Value |
|---|---|
| Age, years, mean ± SD | 56.89 ± 17.22 |
| ≥ 65 yr, N (%) | 66 (63.5) |
| < 65 yr, N (%) | 38 (36.5) |
| Sex | |
| Male, N (%) | 59 (56.7) |
| Female, N (%) | 45 (43.3) |
| Weight, kg, median [IQR] | 58.9 [52.9, 68.3] |
| Height, cm, mean ± SD | 164.01± 9.63 |
| BMI, kg/m2, mean ± SD | 22.41± 4.04 |
| CLCr, mL/min/1.73 m2, median [IQR] | 97.7 [70.8, 126.2] |
| ≥ 60 mL/min/1.73 m2, N (%) | 84 (80.8) |
| < 60 mL/min/1.73 m2, N (%) | 20 (19.2) |
| CLCr, mL/min median [IQR] | 101.1 [74.7, 136.8] |
| ≥ 60 mL/min, N (%) | 86 (82.7) |
| < 60 mL/min, N (%) | 18 (17.3) |
| Target vancomycin trough concentration*, N (%) | |
| 10-15 mg/L | 25 (34.0) |
| 15-20 mg/L | 79 (76.0) |
| Pathogen | |
| Staphylococcus aureus | 19 (18.3) |
| MIC 1 mg/L | 15 (78.9) |
| MIC ≤ 0.5 mg/L | 4 (21.1) |
| Coagulase negative Staphylococcus | 13 (12.5) |
| MIC 2 mg/L | 2 (15.4) |
| MIC 1 mg/L | 7 (53.8) |
| MIC ≤ 0.5 mg/L | 4 (30.8) |
| Enterococcus | 17 (16.3) |
| Vancomycin resistant Enerococcus | 4 (3.8) |
| Vancomycin susceptible Enterococcus | 13 (96.2) |
| Other gram positive bacteria | 5 (4.8) |
| Gram negative bacteria | 10 (9.6) |
| Not detected | 40 (38.5) |
| Indication of vancomycin treatment, N (%) | |
| Bacteremia/Sepsis | 35 (33.7) |
| Neutropenic fever | 12 (11.5) |
| Pneumonia | 10 (9.6) |
| Bone/joint infection | 9 (8.7) |
| Post-operation wound infection | 7 (6.7) |
| Intra-abdominal infection | 6 (5.8) |
| Catheter related blood stream infection | 5 (4.8) |
| CNS infection | 5 (4.8) |
| Skin infection | 3 (2.9) |
| Infectious endocarditis | 2 (1.9) |
| Others | 10 (9.6) |
| Total | 104 |
Data are presented as mean±standard deviation, median (interquartile range), or number (percentage).
Table 2
Total failure rate, subtherapeutic AUC rate, and supratherapeutic AUC rate of candidate Ctrough target range




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download